Assessing the Suitability of a Green Solvent for GPC and TREF Analyses of Polyethylene
LCGC North America
This study presents results of gel permeation chromatography (GPC) and analytical temperature rising elution fractionation (TREF) of polyethylene in butylal (dibutoxymethane, a halogen-free solvent).
The study presents results of gel permeation chromatography (GPC) and analytical temperature rising elution fractionation (TREF) of polyethylene in butylal (dibutoxymethane, a halogen-free solvent). A comparison is made with the results obtained using 1,2,4-trichlorobenzene showing that changing the solvent to butylal does not require any modification of the existing GPC instruments and has additional advantages such as lower toxicity, increased detector signal for polystyrene standards, and broadening domain of crystallization temperatures for TREF.
The molecular structure of polyethylene (PE) is usually analyzed by two techniques: gel permeation chromatography (GPC) and temperature rising elution fractionation (TREF).
GPC separates the macromolecules according to their hydrodynamic volume and allows for a measurement of the molecular weight distribution (MWD). TREF separates semicrystalline polymers as a function of their melting temperature in solution and allows for the evaluation of short chain branching distribution (SCBD).
In both cases, the samples are analyzed in dilute solutions obtained in chlorinated solvents as dichlorobenzene or trichlorobenzene. Usually, the polymer is separated in fractions using a set of columns and the concentrations of the eluted fractions are measured with differential refractive index (dRI) detection. The resulting chromatograms are converted in MWD or SCBD by calibrating the method with standards.
The solvent 1,2,4-trichlorobenzene (TCB) is frequently used for these analyses, mainly because of the following properties:
- Its boiling point is 214 °C, allowing the melting and dissolution of all types of polyethylenes.
- The differential index of refraction (dn/dc) of polyethylene solutions is -0.1 mL/g, which gives good signals in dRI detection for injected concentrations as low as 1 mg/mL.
- Polystyrene is soluble in TCB, which offers the possibility to use the polystyrene–divinylbenzene gel columns and calibrate the GPC systems with narrow standards.
However, TCB also has some disadvantages:
- Its freezing point is 17 °C, close to room temperature, so precautions have to be taken to avoid TCB crystallization, which can damage the GPC column gel and GPC detectors.
- Polystyrene solutions have a dn/dc of 0.05 mL/g, which renders the GPC calibration difficult, especially for low molecular weights of standards obtained by butyl lithium induced anionic polymerization, in which the residual butyl part of initiator with a high negative dn/dc further diminishes the peak intensity.
- It has a relatively high toxicity, causes skin irritation, and is labeled as very toxic to aquatic life with long-lasting effects.
Because of environmental concerns there is a continuous trend toward the replacement of chlorinated solvents with solvents that have a lower toxicity, mostly in the cleaning industry (1). In a recent application patent (2), acetals were described as a valuable replacement for perchlorethylene or trichloroethylene for dry cleaning of textile, leather, or fur goods. In addition to dissolving a large spectrum of compounds, acetals produced by reactions between alcohols and aldehydes can be prepared from renewable or potentially renewable alcohols. With regard to their toxicity, only a few studies are available, but acetals are expected to show very good health and environment profiles and are also biodegradable.
In this study, we show the potential of using butylal in GPC and TREF analyses of polyethylenes. We chose this acetal because of its high boiling point (180 °C), low volatility, and ability to dissolve polystyrene. The evaluation was performed by comparing the results obtained with TCB and butylal on the same samples with the same GPC and analytical TREF systems and methods.
Experimental
Solvents
We used 1,2,4-trichlorobenzene (TCB, Spectropure dry, Biosolve Chimie) and dibutoxymethane (butylal, ultra-pure grade, Lambiotte & Cie, CAS 2568-90-3) for GPC and analytical TREF analyses.
Samples
Polyethylene standard (SRM 1475a, molecular weight [MW] = 52,000 g/mol, polydispersity = 2.9, National Institute of Standards and Technology [NIST]) and a narrow distribution polystyrene standard (MW = 135,000 g/mol, polydispersity = 1.1, Agilent Technologies) were analyzed by GPC. Four commercial metallocene polyethylenes with narrow short-chain branching distributions and the following densities were analyzed by analytical TREF: 0.923, 0.934, 0.947, and 0.955 g/cm3.
GPC Apparatus and Method
The GPC was performed on a Waters GPC 2000 gel-permeation chromatograph (Waters Corporation). The samples were dissolved at 160 °C to obtain solutions with concentrations between 0.1 and 1.0 mg/mL. A 300-µL volume of the hot solution was injected in a 300 mm × 7.5 mm PLgel 10 µm Mixed B column (Agilent Technologies). The flow rate was 1 mL/min, and the temperature of injector, column, and detector was 145 °C. The chromatograms were recorded using the built-in differential refractive index detector. For the analysis of polyethylene in TCB, because of its negative dn/dc, an inversed polarity of detector was selected.
Analytical TREF Apparatus and Method
The analytical TREF was performed using a GPC instrument (Waters GPC 2000 gel-permeation chromatograph), connected with an Agilent GC 6890N oven (Agilent Technologies). The samples were dissolved at 160 °C to obtain solutions with concentrations of 0.5 and 1.0 mg/mL. A volume of 300 µL of the hot solution was injected at 150 °C in a 30 mm × 4.6 mm stainless steel column packed with metallic wires as described in our patent (3). The column filled with solution was fast cooled (1 °C/min) to 100 °C, and then was further cooled to 30 °C with a cooling rate of 0.1 °C/min. After the cooling step, the polymer was eluted from the column using a flow rate of 0.2 mL/min. Before starting the heating from 30 °C to 150 °C with a heating rate of 1 °C/min, the column was maintained at 30 °C for 30 min. The concentrations of the eluted fractions were measured using the built-in differential refractive index detector of the GPC system. A temperature shift of 3.2 °C because of the time delay between the oven and detector, was measured for both solvents using the method described in a previous paper (4) by eluting the polyethylene with a density of 0.947 g/cm3 at different heating rates.
From the practical point of view, replacing the solvent with butylal in the high-temperature GPC and analytical TREF systems running on TCB is extremely easy. Actually, no temperature modification has to be done, the butylal is simply pumping at 1 mL/min to replace the TCB from the column and tubing. After replacing TCB, the dRI detector has to be purged for about 30 min. After purging the detector, the system has to be equilibrated at 1 mL/min as usual, by recirculating butylal for a few hours, before starting the analyses. Because we wanted to provide clear information on the expected results with butylal, apart from using a different offset in Figures 2 and 3 for the curves in butylal, all chromatograms are presented as obtained, without smoothing or drift corrections.
Results and Discussion
Figure 1 is an overlay of the GPC chromatograms of the polyethylene sample SRM 1475a injected at three concentrations (0.25, 0.5, and 1.0 mg/mL) using TCB and butylal. At each concentration, the two chromatograms are similar, the peak areas of butylal chromatograms being about 20% higher than the ones in TCB.
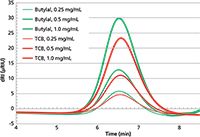
Figure 1: GPC chromatogram overlay of the PE SRM 1475a injected at different concentrations in TCB (red curves) and butylal (green curves).
For numerical comparison, the average molecular weights were calculated in both solvents using a calibration obtained with 12 polystyrene standards and the Mark-Houwink parameters indicated by ASTM D6474 (the standard test method for determining molecular weight distribution and molecular weight averages of polyolefins by high temperature gel permeation chromatography):
KPS = 19 × 10-3 mL/g; αPS = 0.655
KPE = 39 × 10-3 mL/g; αPS = 0.725
The obtained number and weight average molecular weights:
Mn,TCB = 17,400 g/mol;
Mw,TCB = 54,700 g/mol
Mn,butylal = 20,800 g/mol;
Mw,butylal = 57,400 g/mol
were in good agreement with the GPC measured values provided by National Bureau of Standards for this reference material:
Mn = 18,310 g/mol; Mw = 53,070 g/mol
Figure 2 is an overlay of the GPC chromatograms of the polystyrene standard injected at three concentrations (0.1, 0.2, and 0.4 mg/mL) using TCB and butylal. The peak areas in butylal are about four times higher than in TCB for the same concentration. The elution time in butylal is longer than in TCB showing that the Mark-Houwink constants are different in the two solvents.
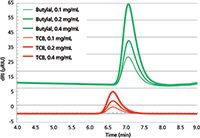
Figure 2: GPC chromatograms overlay of the PS standard with a molecular weight of 135,000 g/mol injected at different concentrations in TCB (red curves) and butylal (green curves).
Stability of the GPC column was checked by running calibration before and after one week under butylal at 145 °C. The calibrations were done with 12 polystyrene standards, covering a molecular range from 160 to 6,870,000 g/mol. A maximum variation in elution times of 0.03 min was recorded between the two calibrations, showing that butylal has no impact on the GPC column packing.
To prove the possibility to use butylal for analytical TREF, we chose to perform analysis on three metallocene polyethylenes with different densities in the same experimental conditions using the two solvents.
Figure 3 is an overlay of the chromatograms of the three polyethylene samples analyzed in TCB and butylal. All samples were injected at a concentration of 0.5 mg/mL. For each polyethylene sample, the shapes of the two peaks are similar, thus producing similar short chain branching distributions. For a polyethylene with a given density, the elution temperature in butylal was 12.3 ± 0.3 °C higher than in TCB. Because the temperature shift between the oven and detector is the same for the two solvents (3.2 °C), the temperature difference between the two solvents is caused by only thermodynamic phenomena.
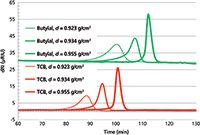
Figure 3: Overlay of six chromatograms (differential refractive index detector signal in function of time) recorded in TCB (red curves) and butylal (green curves) for three different polyethylenes with densities of 0.923, 0.934, and 0.955 g/cm3.
Conclusion
GPC and TREF analyses of polyethylene performed using butylal show similar results compared to TCB, thus becoming a promising replacement for the traditionally used chlorinated solvents.
In addition to a lower toxicity, this halogen-free solvent also has the following advantages:
- A freezing point of -58 °C, much lower than TCB, broadening the domain of temperatures available for TREF.
- Polystyrene–butylal solutions have a dn/dc value that is four times higher than that of polystyrene–TCB solutions, increasing the accuracy of the GPC calibration.
The only possible disadvantage when compared to TCB is the higher flammability of butylal (flash point close cup of 62 °C instead of 99 °C for TCB). This drawback should not be a major concern because highly flammable solvents such as tetrahydrofuran (with a flash point close cup of -14.5 °C) are commonly used in chromatography laboratories and with commercial GPC instruments.
This first study shows the potential use of butylal in GPC and TREF analyses of polyethylenes. Work is in progress to measure the Mark-Houwink parameters for polystyrene and polyethylene in butylal and evaluate the use of this solvent on a broader type of polyolefins.
References
(1) "Assessment to Alternative to Perchloroethylene for the Dry Cleaning Industry," Methods and Policy Report No. 27, June 2012 (Toxic Use Reduction Institute [TURI], University of Massachusetts, Lowell).
(2) M. Seiter, C. Meyer, and H. Eigen, German patent application WO 2010/149521 A1, 29 December 2010.
(3) A.G. Boborodea, C. Bailly, D. Daoust, A. Jonas, and B. Nysten, US patent 7,389,678, 24 June 2008.
(4) A. Boborodea and A. Luciani, LCGC North Am.31(5), 414–418 (2013).
Adrian Boborodea and André Luciani are with Certech ASBL in Seneffe, Belgium. Direct correspondence to:adrian.boborodea@certech.be

Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.












