Anatomy of a Contemporary LC System
LCGC North America
In the inaugural installment of his new column, LCGC veteran Michael Swartz provides an overview of some of the newer LC technology used in contemporary LC laboratories.
Liquid chromatography (LC), defined as any chromatographic procedure in which the moving phase is a liquid (1), has proven to be the predominant technology used in laboratories worldwide during the past 30-plus years. During this time, the field of LC has seen many new developments, all the way from the pump to the recording of the data. Keeping pace with these technological developments, the term LC has also morphed, at times, being referred to as high "pressure" and high "performance", high "speed," and "modern" LC. However, all still have their origins in the early days of LC (2,3).

Michael Swartz
With the relatively recent introduction of columns using sub-2-µm particles, technology seems to be driving the use of the word pressure more and more. Smaller particle sizes increase the pressure drop across the column, giving rise to what today is called "ultra" or "very" high pressure LC, and contemporary LC component and system technology has had to adapt to take full advantage of the increased resolution, speed, and sensitivity afforded by the drop in particle size (4,5). However, regardless of what it is called, today's chromatography laboratory is really concerned with performance, measured as throughput, cost per analysis, or some other metric, and if it is delivered at 1000 psi or 20,000 psi, it really doesn't matter. It is more about what solves the problem, or provides the answer, in as efficient a way as possible. So in that context, in this inaugural column, I'll look at an overview of some of the newer LC technology, and in future columns, I will delve into a few more specifics concerning what constitutes the anatomy of a contemporary LC system.
System Architecture
Contemporary LC systems contain the same basic components as those dating back to the 1970s and 1980s: a pump, an injector, a column, a detector, and a data-recording device. However, the way in which these components are connected and interact has changed dramatically over the years. Contemporary LC systems can be either modular or integrated in design, and use high-pressure or low-pressure solvent mixing. Modular systems are constructed of individual components that can be mixed and matched depending upon the application. There are some advantages to a modular system architecture: the components can be selected based upon need and performance (for example, the most sensitive detector for a particular application) and can be serviced easily with minimal downtime by replacing a module in the event of a failure. However, there are some potential disadvantages to a modular architecture: variable system volumes, system-to-system variability, multiple fluidic and data–control connections, and regulatory issues (for example, instrument qualification when modules are changed) all need be considered. Integrated systems consist of components, primarily the pumps and injector, that can be operated from a single control source and user interface. In the integrated format, if one component goes down, the whole system is affected. That likelihood might be diminished over a modular system as there is less complexity and fewer power supplies, check valves, seals, and so forth. System-to-system variability also is diminished, as each system can be preconfigured identically at the manufacturer.
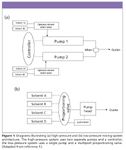
Figure 1
Solvent Management
System architecture can be classified further by how the solvents in the mobile phase are blended, as illustrated in Figure 1. Traditional high-pressure systems use two or more pumps to blend solvents under high pressures. A separate controller is used to alter the flow rate to blend different proportions of solvent or to generate gradients, using external mixers. While the system has the disadvantage of the cost and maintenance of multiple pumps, high-pressure systems typically have lower system (gradient delay) volumes, important when using smaller diameter, sub-2-µm particle columns, and fast LC techniques. High-pressure systems are usually binary systems, although optional solvent selection valves can grant access to more than one solvent at a time per pump. In low-pressure designs, a single pump draws the solvent through a multiport proportioning valve. Software algorithms control and time the opening of the ports with the pump stroke under microprocessor control to blend the solvent or generate a gradient in the pump head under low pressure. Degassing, either by helium sparge or membrane modules, is required to prevent outgassing during solvent blending. The simplicity of a single pump is certainly an advantage, but the pump head and other downstream components contribute to a typically larger system or gradient delay volume than that found in high-pressure systems. Low-pressure systems offer more flexibility in solvent selection than high-pressure systems and can be used to generate different mobile-phase compositions online (as opposed to premixing), adding more flexibility during method development. In addition, in low-pressure systems, any solvent volume change that might occur during mixing is accomplished before the solvent is pressurized; therefore, flow-rate changes that might result from this effect in high-pressure systems that do not precompress the solvent are not a problem with low-pressure systems. Many of the advantages of low-pressure mixing systems, and the disadvantages of high-pressure mixing systems, are offset by the necessity of using low-dispersion (low-volume) systems like those necessary to make the best use of the small-particle columns on the market. Low-pressure mixing systems have topped out at about a maximum of 6000 psi, while high-pressure mixing systems, with about ~10x lower gradient delay volumes, are available that exceed 15,000 psi capabilities.
Sample Management
Injectors and autosamplers today do a lot more than simply introduce the sample to the column, they are true "sample managers." They must be able to handle sample formats ranging from vials to 96- or 384-well microtiter plates and have integrated–automated wash routines to reduce carryover, a necessity due to the high sensitivity requirements of many of today's analyses. Injection volume flexibility, the ability to cool samples for added stability, the ability to compensate for sample viscosity, and multiple vial–carousel–plate access are also desirable traits. Many sample-management systems also can perform sampling routines for automated reference peak addition or precolumn derivatization procedures.
Another important sample-management factor to keep in mind is what I refer to as injection overhead, or all of the time spent doing things outside of the actual chromatographic run. Things like positioning the sample for injection, aspirating and injecting the sample, and wash routines all add time to the overall sample injection-to-injection cycle time. In traditional LC, with run times of 20–30 min or more, injection overhead times are not that significant. However, with run times now on the order of 1–2 min, such as those encountered using sub-2-µm column particles, a minute or more of injection overhead can affect injection-to-injection cycle time significantly, and consequently throughput, by as much as 50%. Contemporary LC systems have reduced the injection overhead by performing common tasks during the chromatographic run in parallel, rather than in series. By handling injection overhead in this manner, injection-to-injection cycle times are not compromised.
Faster injection-to-injection cycle times also have an impact on the instrument and laboratory staff utilization and workflow paradigm. With an inject-to-inject cycle time of 2–3 min, running two shifts unattended overnight (16 h) requires a sample capacity of 320–480 samples, a level only a few contemporary LC systems can provide. In addition, a workflow shift to more people actually preparing samples and compiling data as opposed to the number of people operating the instruments, might have to evolve.
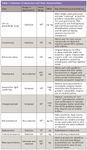
Table I: Common LC detectors and their characteristics
Detectors
There is no one detector that can be employed for all LC separations, and consequently, many different types of detectors are available for LC. They are listed in Table I along with their key attributes and limitations. (Note: I've included mass spectrometry [MS] on the list, but I won't address advances in MS technology here as it is the subject of other columns in LCGC on a routine basis.) The list of detectors for LC really hasn't changed much recently, but significant improvements have been made to extend the linear range, improve sensitivity, and/or their ease-of-use in contemporary LC systems. For example, the utility of UV detection has been extended by the use of photodiode arrays. Photodiode array detection (PDA or DAD) can provide spectra of eluted peaks that can be used to aid peak identification and to monitor for co-elutions, helpful during method development. PDA detectors also can serve as a multiwavelength UV–vis detector. Early PDA detectors were not as sensitive as single-wavelength UV detectors, but the gap has been closing in recent years thanks to a new detector cell type, as illustrated in Figure 2. This detector cell consists of a light-guided flow cell equivalent to an optical fiber. Light is transferred efficiently down the flow cell in an internal reflectance mode that still maintains a 10-mm flow-cell path length with a volume of only 500 nL, extending the path length while maintaining low dispersion. An example of the sensitivity and the dynamic range that is possible with a PDA detector is shown in Figure 3 for an active pharmaceutical ingredient (API) impurity at the 0.01% level.
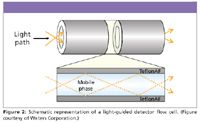
Figure 2
Another example of an improvement in detector technology is the advent of universal-type response detection methods that use nebulizer technology, like evaporative light scattering detection (ELSD) or charged aerosol detection (CAD). In both ELSD and CAD, the HPLC column eluent is first nebulized with a nitrogen (or air) carrier gas to form droplets that are then dried to remove mobile phase, producing analyte particles. ELSD measures the scattered radiation of a laser beam caused by the particles in the flow path. In CAD, the primary stream of analyte particles is met by a secondary nitrogen stream that is positively charged as a result of having passed a high-voltage, platinum corona wire. The charge transfers diffusionally to the opposing stream of analyte particles, and is further transferred to a collector where it is measured by a highly sensitive electrometer, generating a signal in direct proportion to the quantity of analyte present. For either ELSD or CAD, chromophores are not required, but compounds must be nonvolatile and volatile mobile phase buffers must be used. A schematic of a CAD system is shown in Figure 3. CAD is somewhat more sensitive and has a wider dynamic range than ELSD and has found many applications in the contemporary LC laboratory (6).

Figure 3
In addition to detector cell technology, improvements in speed or data rate also are required in contemporary LC systems, particularly when using sub-2-µm particle columns. One rule of thumb says that to define a peak for good integration adequately, 15–20 points should be collected across the peak. For a peak 20–30 s wide, a data rate of 1 Hz might suffice. But for peaks obtained from sub-2-µm particle column separations, peaks on the order of 1-s wide can be obtained, and data rates upwards of 10–100 Hz might be required, as shown schematically in Figure 5. Figure 6 depicts how the data rate can affect resolution and sensitivity for the separation of four organic acids on a sub-2-µm column particle on a system otherwise optimized on this scale.
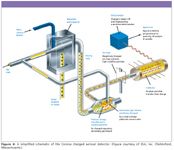
Figure 4
Chromatography Data System
The term chromatography data system or software (CDS) is really a misnomer when referring to a contemporary LC system, because they do so much more than just collect data. The CDS often controls the entire system, performs complex chromatographic (for example, resolution, plate count, and tailing factor) and statistical (for example, %RSD, regression analysis, and t-test) calculations, as well as reporting in customizable formats. Gone too are the days of flat-file architecture. Relational databases with time and date stamps, audit control, and customizable user interfaces and access are the norm today. The databases allow the data to be accessed, searched, and compiled across multiple systems or even locations (7). Database technology also makes it possible to tie all of the "metadata" (all of the related sample data, including the., instrument method and analyst identification) together.

Figure 5
While the CDS now is also tapped into every component of the LC system for control, monitoring, and diagnostics, it also is often connected to the outside world as well. Remote access and diagnostics services available from many vendors provide proactive service support to maximize instrument availability and minimize downtime costs.
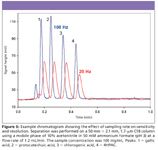
Figure 6
Specialized LC Systems
New areas of research have given rise to specialized LC systems, configured to meet challenges imposed by the types of molecules analyzed, the sensitivity required, the amount of sample available (or desired), and the amount and type of information needed. Hyphenated systems, such as LC–MS, LC–MS-MS, LC–nuclear magnetic resonance (NMR), or even LC coupled with other spectroscopic detectors like infrared or atomic adsorption, can provide a wealth of information about the composition and structure of an analyte of interest. Capillary systems, also used as "inlets" in a hyphenated system, can be used to enhance mass sensitivity in sample-limited situations.
Some life science applications, for example, proteomics (the study of a full set of proteins encoded by a genome), involve samples that are too complex for a single LC analysis. In these instances, two-dimensional systems are used commonly to fractionate the samples to increase peak capacity (8–10). In these systems, two orthogonal methods (for example, different modes of chromatography, reversed phase and ion exchange, or two different pHs) are used to fractionate a sample in the first dimension and then analyze the fractions in the second dimension. In this manner, peak capacities can be extended from as little as 200–300 to as much as 10,000 or more, providing a wealth of information for the characterization of these very complex samples.
The list of specialized systems also includes inert systems, typically constructed of titanium or PEEK. Inert systems are configured for biocompatibility in the case of life-science applications, or for contaminate free mobile-phase operation in the case of ion chromatography or high-sensitivity electrochemical detection. There are also many application-specific systems available, including those for carbamate analysis, amino acid analysis, and gel permeation–size exclusion chromatography to name a few.
Conclusion
Until about five years ago, any existing LC method could be run on any LC system, of any vintage, with only minor modifications. The demands of today's laboratory, with a higher number of more complex samples, requires newer technology offering low dispersion and noise, high speed, and sensitivity. Whether referred to as a pump and injector or as a solvent and sample management system, high performance or high pressure, the current pace of technicological advances will keep LC in the forefront of the laboratory for years to come.
Michael Swartz "Innovations in HPLC"Editor Michael E. Swartz is Research Director at Synomics Pharmaceutical Services, Wareham, Massachusetts, and a member of LCGC's editorial advisory board. Direct correspondence about this column to "Innovations in HPLC," at lcgcedit@lcgcmag.com.
References
(1) L.R. Snyder and J.J. Kirkland, Introduction to Modern Liquid Chromatography, 2nd ed. (Wiley and Sons, Inc., Hoboken, New Jersey, 1979), p. 1.
(2) L.R. Snyder and J.J. Kirkland, Introduction to Modern Liquid Chromatography, 2nd ed. (Wiley and Sons, Inc., Hoboken, New Jersey, 1979), pp. 6–8.
(3) E.L. Johnson and R. Stevenson, Basic Liquid Chromatography (Varian Associates, Palo Alto, California, 1978), p. 1.
(4) "The Quest for Ultra Performance in Liquid Chromatography — Origins of UPLC Technology," Waters Corporation. See: http://www.waters.com/waters/partDetail.htm?cid=511539&id=38628&ev=10120661&locale=en_US
(5) "Beginners Guide to UPLC — Ultra-Performance Liquid Chromatography," Waters Corporation. See: http://www.waters.com/waters/partDetail.htm?cid=511539&id=38629&ev=10120684&locale=en_US
(6) M.E. Swartz, M. Emanuele, A. Awad, and D. Hartley, in Advances in HPLC, LCGC Supplement, 40–48 (April 2009).
(7) R.P. Mazzarese, Handbook of Pharmaceutical Analysis by HPLC, S. Ahuja and M.W. Dong, Eds. (Elsevier, Amsterdam, The Netherlands, 2005), p. 581.
(8) R.E. Murphy, M.R. Schure, "Instrumentation for Comprehensive Multidimensional Liquid Chromatography," in Multidimensional Liquid Chromatography: Theory and Applications in Industrial Chemistry and the Life Sciences, S.A. Cohen and M.R. Schure, Eds. (Wiley and Sons, Inc. Hoboken, New Jersey, 2005).
(9) S.A. Cohen and S.J. Berger, "Multidimensional Separation of Proteins with On-Line Electrospray Time-of-Flight Mass Spectrometric Detection," in Multidimensional Liquid Chromatography: Theory and Applications in Industrial Chemistry and the Life Sciences, S.A. Cohen and M.R. Schure, Eds. (Wiley and Sons, Inc., Hoboken, New Jersey, 2008).
(10) M.R. Schure, Advances in HPLC, LCGC Supplement, 8–15 (April 2009) .
Reversed-Phases for LC Deliberately Doped with Positive Charge: Tips and Tricks for Effective Use
May 13th 2025In this month's edition of LC Troubleshooting, Dwight Stoll and his fellow researchers discuss both the benefits (improved peak shape/loading) and challenges (excessive interaction) associated with charge-doped reversed-phase (RP) columns for both analytical and preparative separations.
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













