Solvent, Chemistry, and Other Myths in LC–MS
LCGC North America
Michael Balogh uses examples such as the recent acetonitrile shortage to examine the topic of "the unknowable" as it pertains to separation science.
Sometimes we can be surprised to find we have overlooked details in areas where we are otherwise so well versed. Then, forced to have another look, find we have opened a wealth of understanding. The art and science of chromatography often seems to offer opportunity for passionate debate. Over the past seven years, since CoSMoS conferences began, the chromatography discussion sessions have been the most highly rated, often inciting the most vigorous debate. A now-famous quote from former U.S. Secretary of Defense Donald Rumsfeld might apply well: "There are known knowns. These are things we know that we know. There are known unknowns. That is to say, there are things that we now know we don't know. But there are also unknown unknowns. These are things we do not know we don't know." (This quote was taken from a press briefing February 12, 2002, on the increasingly unstable situation in Afghanistan.)

Michael P. Balogh
A recent discussion at CoSMoS 2009 (Boston, Massachusetts, see www.CoSMoScience.org) proves Rumsfeld's observations aptly describe the state of much of the underlying, and often misguided, assumptions we bring to bear when applying our understanding of chromatography to liquid chromatography–mass spectrometry (LC–MS) practice. The inherent value of the charter principle of CoSMoS since its founding in 2003 is crafting open discussion workshop sessions to encourage a free exchange of ideas, leading to enhanced practical knowledge.
One might, for example, approach LC–MS with a wealth of condensed phase knowledge based upon years of chromatography practice and accept the prospect that protonation (and therefore sensitivity) is enhanced at least in positive-mode electrospray by ensuring the analyte is more basic than the solvent. While at odds with accepted practice previous to widespread MS use, adding a volatile modifier such as acetic acid in place of the usual, less-volatile mobile phase components found in LC practice becomes commonplace. In many ways, our solvents of choice have followed similar paths.
Marketing literature — for example, a trade column written by a scientist employed by industry — while typically designed to be useful, can nonetheless be biased, seeming to further confound understanding. The same can be true for conference presentations. A speaker who brings a lifetime of interest, effort, and achievement in a given area will necessarily color the presentation and typically place too much burden of preparation and prior knowledge on listeners much less familiar with the topic. The members of the CoSMoS planning board believe providing a platform for ideas is, of itself, not enough and those ideas need "rounding out" if not debunking outright.
For example, the commercial success of a new technology such as ultrahigh-pressure liquid chromatography (UHPLC) is often debated by what in political terms might be called the "loyal opposition." Such a debate requires a neutral, public arena for discussion on the merits with as many different facets of the argument as possible in full view. Arguing the merits of even common practice such as heating the mobile phase or the number of plates one needs to achieve, while clearly important and enduring topics, requires more than a single voice to achieve understanding. I stumbled upon a comment when preparing material on monolithic columns from the thesis of Jennifer Smith (1), someone I met briefly when she was a student of Harold McNair (Virginia Polytechnic Institute, Blacksburg, Virginia). "The term 'fast HPLC' is a relative one. Analysis time of itself is a poor measurement of chromatographic performance; rather the important parameter is the number of peaks separated per unit time. For example, a 10-component run in 10 min is more time efficient than a two-component run in 10 min. Nevertheless, it should be noted that the terms "fast LC,", "fast HPLC," "high-speed HPLC," and "ultrafast HPLC" are commonplace in the literature without formal definition."
Smith's observation conveys needed clarity. The work of Uwe Neue and others (2) describes the possibility of extremely efficient separations under fast-flow conditions when pressure is not a factor. Today, when continuing this discussion, we also should be cognizant of work done by Jorgenson, Lee (3), and others who advanced other time-honored practices such as high temperature along with high pressure because it benefits our analytical interests. Lee's work published in 2003 shows an initial 4-min separation for an eight-component barbiturate mixture with a uracil marker performed at 10,000 psi and 220 °C shortened to less than 40 s at 35,000 psi and 800 °C. Of course, when the mixture is too complex, simply making a run faster is clearly not of value nor is this impressive capability within reach of most practitioners without specialized equipment.
The value of conferences such as CoSMoS lies in discussing topics and concepts that are often difficult to comprehend. The conference is not the byproduct of a gathering but is instead the primary purpose for practitioners to gather. This year, for instance, attendees were fortunate to hear Georges Guichon and Pavel Jandera speak on the advantages and costs of two-dimensional chromatography (the presentations are available to download at http://www.cosmoscience.org/archive_2009.htm). Both speakers were featured at other conferences as well but being a small discussion-focused event, CoSMoS attendees also got to participate in the ensuing discussions.
The Acetonitrile Crisis
Expectation can cause otherwise rational folk to form opinions not supported by fact. An enlightening exchange organized by CoSMoS board members Bill Farrell (Pfizer, La Jolla, California) and Wolf Goetzinger (Amgen, Cambridge, Massachusetts) underscores our appreciation of a powerful solvent we have come to rely upon.
Many readers can appreciate the recent concerns over whether a shortage in the market of acetonitrile will inhibit our practice. In their session, Jerry Richard, president of Purification Technologies Incorporated (PTI, Chester, Connecticut) described both the process to acquire and to purify acetonitrile to put matters in perspective. PTI purifies acetonitrile, a relatively small (3–5%) byproduct of the acrylonitrile synthetic process to the level of purity, required for the pharmaceutical industry and analytical practice. A common method called the Sohio Process (propylene ammoxidation) yields acetonitrile as a coproduct, which is 60–80% pure, containing a host of impurities, including 15–25% water along with acetone, hydrogen cyanide, propionitrile, toluene, and others. Purity of 99.9% is sufficient for the major markets. Use by analytical interests, which can tolerate only trace impurities, requires further processing but accounts for a very small fraction of the market (Figure 1). Pharmaceutical processes (37% of the total) and synthesis (39%) are large by comparison but require less refined product.
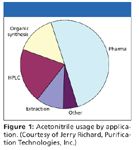
Figure 1
China and Russia more than equal the US in production of acrylonitrile (Figure 2) but in some cases the industrial source chooses to not commit the 'waste' or co-product of their primary process to a company like PTI. China reduced acrylonitrile production in 2008 in favor of improving the environment for the Olympics. In addition, against a backdrop of reduced production by a hurricane-damaged refinery on the U.S. gulf coast, the automobile market faltered and the demand for plastics among other primary uses of the chemical decreased.
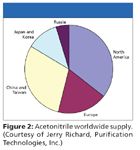
Figure 2
By August 2009 when the annual CoSMoS conference was held, the acetonitrile shortage apparently already had subsided (Figure 3). Recently Richard informed me that "The bulk acetonitrile market changed significantly over the summer. Now, acetonitrile is readily available and the Asian producers are working to import material into the United States. INEOS is announcing that they have improved acetonitrile yields due to a new technology. On the other hand, the Lucite facilty in Texas is being closed, no longer producing acetonitrile. Prices are falling but have not reached preshortage levels and I doubt they will."

Figure 3
"The demand for acetonitrile is slow at this time. That seems partly a seasonal effect, partly some inventory or production planning effects and partly due to the fact that users have gone to other solvents. At this time, we are not sure the magnitude of each factor. However, we do expect demand to improve into 2010."
Aftermath of the "Crisis"
Though perhaps a true "crisis" did not materialize, the shortage prompts us to reflect on critical aspects of our work. The success over many years of reversed-phase LC has increased the utility of acetonitrile. From Goetzinger and Farrell's notes:
- Analytical separations use relatively small amounts of acetonitrile.
- A 5–10 min LC analysis consumes 5–20 mL which even at $100/L is less than $2 per sample.
- Preparative separations using more than 200 mL per sample is an issue.
Goetzinger and Farrell also speculate that a normal-phase technique like supercritical fluid chromatography (SFC), in which the preferred solvent is methanol, will attract a lot of attention in the future despite the nontrivial process of transferring from one separation mechanism (reversed-phase LC) to SFC. In the prevailing opinion, it seems SFC today might be where high performance liquid chromatography (HPLC) was in the 1980s. So SFC stands as an impressive prospect.
Smaller Internal Diameter Columns
Another commonly raised issue is the various benefits — and tradeoffs — of using smaller internal diameter columns. Solvent consumption is proportional to the square of the column internal diameter. Peak volume also decreases to the same extent, which is clearly a problem for concentration-dependent detectors, but in view of electrospray's dependence upon electrohydrodynamics, not so much of a problem for MS detection.
Reducing the flow rate (overall or by postcolumn splitting) has long been a mainstay practice in LC–MS to improve sensitivity. However, any impediment to a well-formed separation (changes in tubing internal diameter, increased tubing length, or junctions in the flow path) will result in dramatic loss of fidelity and sensitivity, even more evident at reduced scale. Mass spectrometers, unlike UV detectors, are mass-sensitive so less solvent requiring vaporization results in a more uniform population of smaller droplets that desolvate to the "magic" 10-µm radius faster, liberating ions more uniformly. If peak width is maintained equivalent in the MS trace as seen in UV, one can see an apparent gain in sensitivity. A reduction of either mass or concentration at peak maximum will, however, result in less than optimum results.
An example of how our thinking can be changed by such disruptions in our normal practice came from a colleague in Japan a few years ago. Jun Yonekubo was developing an analytical separation to determine additives in polymers. The typical approach to LC–MS analysis of such antioxidants requires using atmospheric pressure chemical ionization (APCI) because they are not amenable to electrospray ionization (ESI). Historically, we needed to change the separation to accommodate the higher flow rate of APCI. Where a 2-mm i.d. column used for ESI might optimize at a flow rate of 0.250 mL/min, the linear velocity for optimum traditional APCI was closer to 1 mL/min. Considering the negative effects on droplet formation — the need for increased desolvation of higher vapor loads — and any gains expected from increased column loading on a 4.6-mm i.d. column quickly disappears. Yonekubo-san found having the freedom to reduce the column diameter and flow rate using the multimode approach of ESCi (a registered trademark for Waters Corporation multimode technology), where APCI is performed under "ESI conditions," also condensed the separated bands at this reduced scale, which favored the MS need for higher signal-to-noise ratios. UV detection can better handle a rather diffuse, broad peak with minimal signal-to-noise ratios because the peak is characterized with contiguous wavelengths. MS response improves as signal is better differentiated from noise because the spectrum for even a well-formed separated band is composed of a wealth of discrete ions.
Speaking with some retrospective in the wake of the acetonitrile event, Goetzinger offered some observations: "Reducing particle diameter from 3 µm to 1.8 µm (with optimized equipment) allows separation time to be reduced by 40%. Assuming you need the same number of plates (that is, going from 50 mm to 30 mm length) the amount of eluent is respectively reduced."
Bearing in mind Smith's comments on "defining fast LC" as increasing information not just speed, Goetzinger remarks "Given the advantages of smaller particles, [UHPLC] is probably something we'll all be doing in a few years since pressure drop is cheap!"
The moderators of the CoSMoS workshop expressed this caveat: Beware of performance losses when moving to smaller internal diameter columns. Practitioners have always been encouraged to place an emphasis on low-dead-volume, well-designed systems. This is certainly true when working at reduced scale. Pay attention to splitting for hyphenated techniques as peak volume is reduced and disrupting the well-formed separation is possible.
Are There Real Alternatives?
Acetonitrile has become the popular solvent of choice due to low viscosity when mixed with water, enabling high flow rates. The high diffusivity of water–acetonitrile mobile phases provides high separation efficiency and little UV absorbance at low wavelengths for good detection limits. Alcohols, ethers, and acetone don't perform comparably. All contribute to high viscosity in aqueous mixtures, leaving only methanol as a viable alternative.
Acetonitrile, due to its elutropic strength, has become a favorite for many applications. Even though one might think an aprotic solvent would not perform well in the electrospray process, there are numerous ways to provide protons at atmosphere as opposed to vacuum ionization processes. Methanol has so far shown little benefit when employed with monolithic columns and mixing methanol with acetonitrile is of limited value as Goetzinger points out, resulting in "issues with reproducibility, long-term stability . . . possibly the worst of both worlds."
Replacing acetonitrile is a question of balancing chromatography performance, pressure and cost. Until the 1990s, methanol was the reversed-phase solvent of choice. When particle diameters decreased from 5 µm to 3 µm, acetonitrile popularity rose accordingly. Methanol requires about 10% higher organic content to produce the same retention factor and selectivity may change. The increased viscosity resulting from using methanol results in increased pressure drop and, typically, a reduced efficiency.
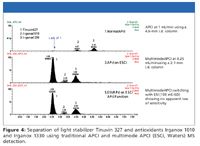
Figure 4
David Masters-Moore (Pfizer, La Jolla, California) makes similar arguments. Investigating alternatives in high-volume purification solvent usage, his group has recently reviewed their practice. Their study compared mixtures of solvents selected to reduce overpressure risks: ethanol, isopropanol, and n-butanol in combination with methanol. Using a simple mixture of parabens, Masters-Moore found 30% butanol and isopropanol and 60% ethanol–methanol mixtures performed most similar to acetonitrile (Figure 5). All had adequate resolution and acceptable pressure gradients. In his estimation, no plumbing, column, or temperature changes were required. The pressure gradients for each mixture were similar to methanol alone.
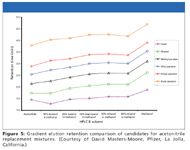
Figure 5
Others have taken a careful look at some of the underlying behaviors and found we sometimes misinterpret what we see (4). Butanol mixtures, for instance, generate more heat than other alcohol aqueous mixtures and measuring the column temperature can show a disparity between the actual temperatures measured not visible in retention changes. So we might not see much need to amend our practice.
As always, I am indebted to those who graciously share their efforts for the benefit of the rest of us. David Masters-Moore is currently a Senior Research Scientist at Pfizer La Jolla. He has provided chromatography and MS expertise for multiple pharmaceutical, natural products, and agrochemical companies. Bill Farrell is Senior Principal Scientist in High Throughput Analysis and Purification, Pfizer Global Research (La Jolla, California) and a director for the Society of Small Molecule Science, which organizes the CoSMoS conference each year. Wolfgang Goetzinger is Director, Research, Molecular Structure, at Amgen (Cambridge, Massachusetts) and a board member of CoSMoS since 2006. Jerry Richard is President of Purification Technologies Inc. (Chester, Connecticut).
Michael P. Balogh "MS — The Practical Art" Editor Michael P. Balogh is principal scientist, MS technology development, at Waters Corp. (Milford, Massachusetts); a former adjunct professor and visiting scientist at Roger Williams University (Bristol, Rhode Island); cofounder and current president of the Society for Small Molecule Science (CoSMoS) and a member of LCGC's editorial advisory board.
References
(1) J.H. Smith, "Chromatographic Properties of Silica-Based Monolithic HPLC Columns," Dissertation, Virginia Polytech Institute, Blacksburg, Virginia, 2002.
(2) U.D. Neue, J.L. Carmody, Y.-F. Cheng, Z. Lu, C.H. Phoebe, and T.E. Wheat, Adv. Chromatogr. 41, 93 (2001).
(3) Y. Xiang, B. Yan, B. Yue, C.V. McNeff, P.W. Carr, and M. Lee, J. Chromatogr., A 983(1–2), 83–89 (2003).
(4) W. Goetzinger, publication in process for 2010.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









