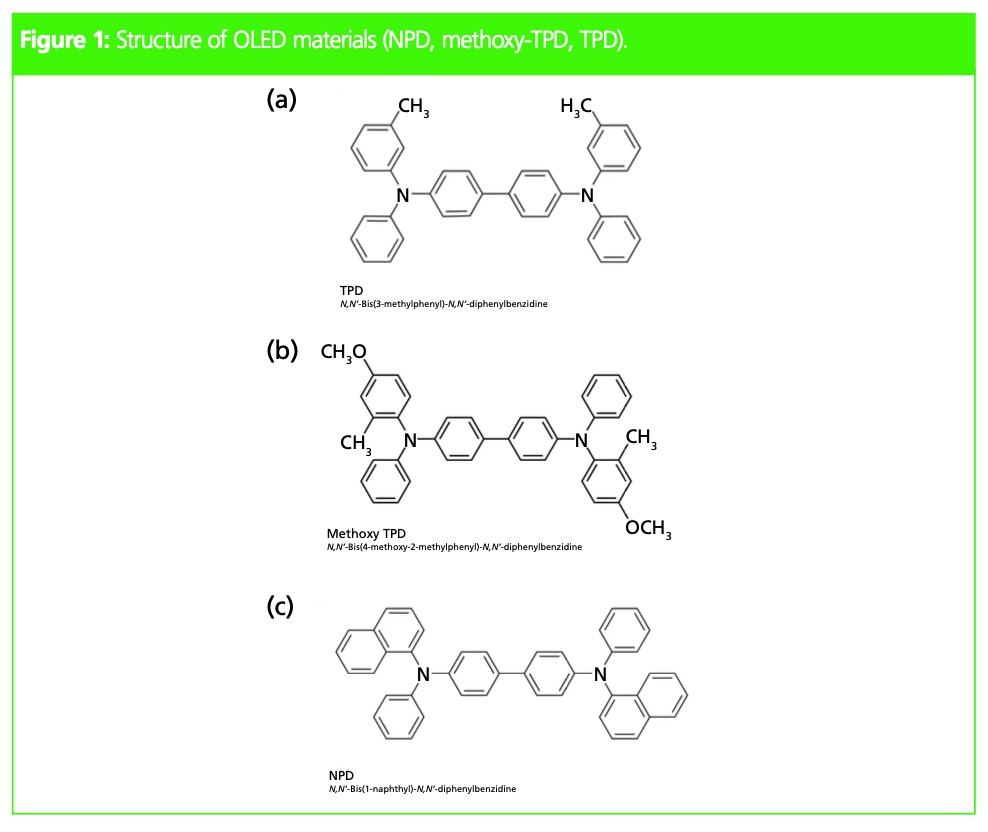
Analyzing the Light of the Future
Organic light-emitting diodes (OLEDs) are widely used to create digital displays. To ensure high-performance products, the luminous efficiency and stability of OLED materials are essential and undergo strict quality control (QC). This article introduces a novel analytical method for the analysis of OLED materials using supercritical fluid chromatography (SFC) as well as purification by large-scale prep SFC. Switching from high performance liquid chromatography (HPLC) to SFC for the analysis of these organic compounds enabled a significant reduction of organic solvent consumption, decreasing both the cost of analysis and the environmental impact. SFC technology offers a valuable alternative to HPLC for analyzing OLED materials.
Organic light-emitting diodes (OLEDs) are a hot topic in the lighting industry, with some calling them “the future of lighting." In addition to being thin, flexible, light, cool to the touch, and not containing blinding light sources, they are sustainable as they significantly reduce power consumption, greenhouse emissions, and decrease non-recyclable waste.
OLEDs include a layer of thin organic (carbon-based) material that can emit light in response to an electric current. Unlike LEDs, OLEDs are manufactured in panels, resulting in diffuse light sources for a wide range of applications. Nowadays, they are widely used to create digital displays in smartphones, televisions, and PC monitors. To ensure high-performance products, the efficiency and stability of OLED materials need to undergo strict quality control (QC) (1).
The luminescent materials are classified as polycyclic aromatic compounds, where effective analytical and preparative methods are required to identify their structure and analyze impurities in high-quality OLED materials (2). This article describes the development of a novel analytical method for the analysis of OLED materials using supercritical fluid chromatography (SFC), as well as purification by large-scale prep SFC.
Method Development
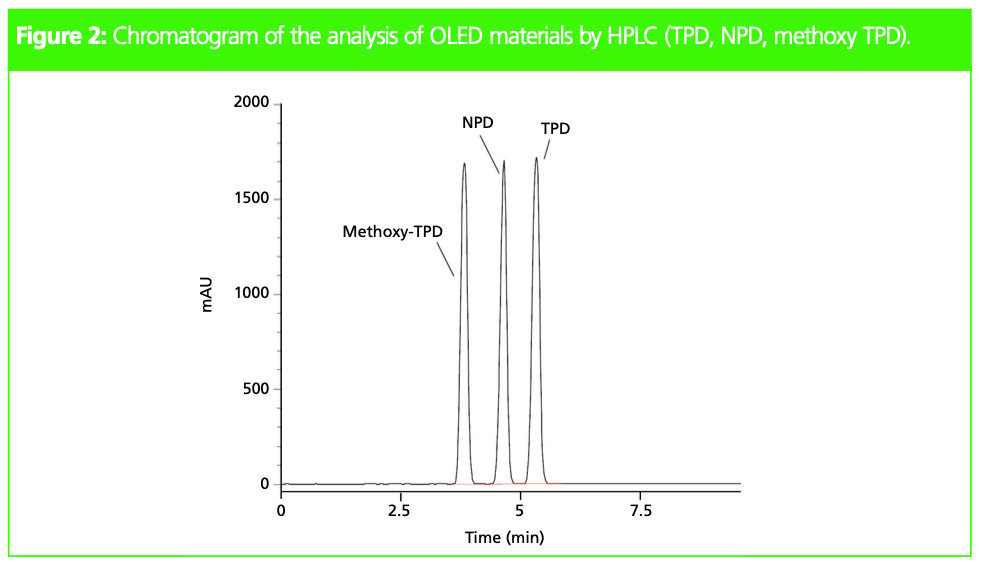
OLED materials (Figure 1) are nonpolar and therefore have low water solubility, which is why conventional high performance liquid chromatography (HPLC) analysis requires a high percentage of organic solvent in the mobile phase. Figure 2 shows an example chromatogram of HPLC analysis of the three OLED materials shown in Figure 1.
Analytical Conditions for HPLC Analysis of OLED Materials
System: Nexera XR UHPLC system (Shimadzu); column: 250 mm × 4.6 mm, 5-μm Shim-pack VP-ODS (Shimadzu); flow rate: 3.0 mL/min; mobile phase: A) 9:1 acetonitrile–water, B) tetrahydrofuran; time program: 5% B (0 min) -> 30% B (10 min) -> 30% B (10.01–15.00 min); column temp.: 40 °C; injection volume: 10 μL; detection (PDA): λ = 350 nm.
As SFC uses nonpolar carbon dioxide (CO2) as the main mobile phase, which is significantly cheaper to acquire and free to dispose of compared with organic solvents, it could be a more cost-effective technique than HPLC for the analysis of these components. In addition, because carbon dioxide is inert, the risk of degradation of the target compounds is lower than in conventional HPLC. To develop a suitable SFC method, a dedicated software tool (the Shimadzu Method Scouting solution) was used to set up a method screening experiment. Six different columns and four organic modifiers were evaluated creating 24 different analytical conditions to be tested. The different stationary and mobile phases under investigation are listed below.
Analytical Conditions for SFC Method Scouting for Analysis of OLED Materials
System: Nexera UC analytical SFC system; columns: 250 mm × 4.6 mm, 5-μm Shim-pack UC-PyE, Diol II, Phenyl, RP, Sil, GIS II (Shimadzu); flow rate: 3.0 mL/min; mobile phase: A) CO2, B) methanol or acetonitrile or acetone or tetrahydrofuran; time program: 2% B (0–1 min) -> 40% B (6–8 min) -> 2% B (8.01–10.00 min); column temp.: 40 °C; injection volume: 5 μL in tetrahydrofuran (250 mg/L); back pressure (BPR): 10 MPa; detection (PDA): λ = 350 nm.
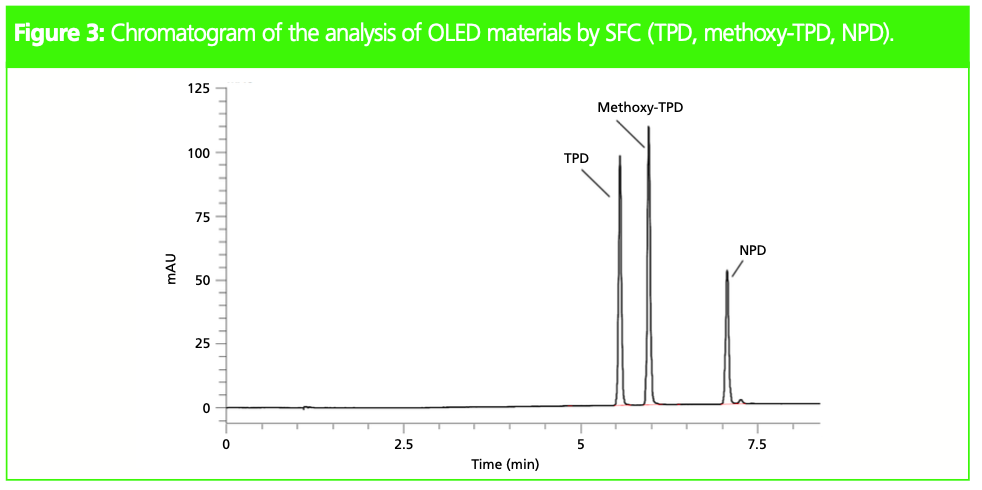
The Shim-pack UC Diol II column and acetonitrile as modifier were identified as suitable separation conditions. The resulting SFC method was used for quantitative analysis of OLED materials (Figure 3).
Preparative SFC Conditions for Purification of OLED Materials
System: Nexera UC prep, preparative SFC system (Shimadzu); columns: 250 mm × 20 mm, 5-μm Shim-pack UC Diol II (Shimadzu); flow rate: 60 mL/min; mobile phase: A) CO2, B) methanol or acetonitrile or acetone or tetrahydrofuran; time program: 2% B (0–1 min) -> 40% B (6–8 min) -> 2% B (8.01– 10.00 min); column temp.: 40 °C; injection volume: 200 μL in tetrahydrofuran (5 g/L); back pressure (BPR): 10 MPa; detection (PDA): λ = 350 nm; make-up flow: 5 mL/ min (tetrahydrofuran).
Results and Discussion
Quantitative Analysis by SFC
Calibration curves for the three analytes of interest were prepared ranging from 1 to 250 mg/L. With R2 > 0.999 and %RSD < 0.7, the method showed excellent linearity and repeatability for all three compounds. Limits of detection (LOD) were determined as 0.13, 0.16, and 0.28 mg/L for TPD, methoxy-TPD, and NPD respectively, while consumption of organic solvent could be reduced by more than sevenfold.
Preparative Purification by SFC: This reduction in organic solvent consumption was even more significant when looking at preparative purification. Upscaling from analytical SFC to a larger scale preparative method was easily achieved utilizing the same column packing material and column length (250 mm), with a wider inner diameter (20 mm) so that only the flow rate had to be increased.
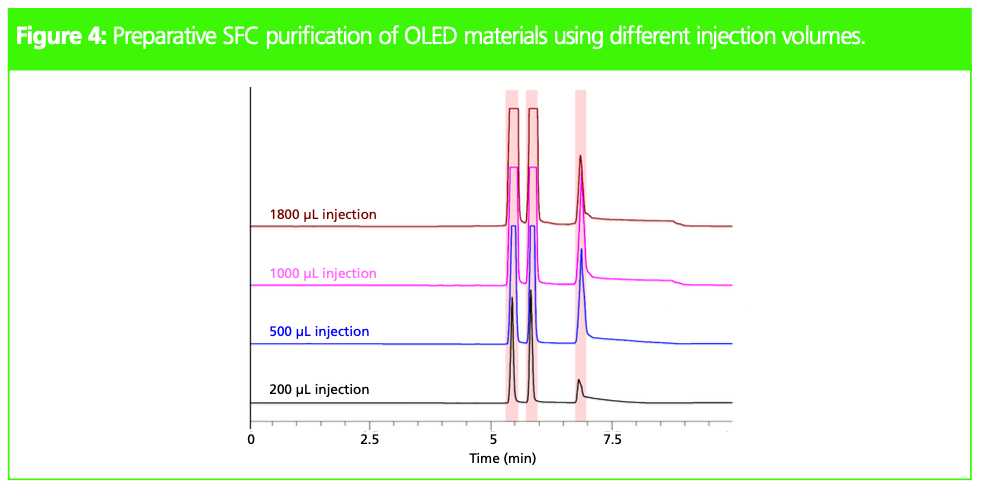
High sample loading is important to achieve a large amount of purified compound. However, peak separation and peak shape can deteriorate when the injection volume is too large. The so-called on-column dilution function to dilute sample solvent during injection can help to reduce the effect of sample solvent and maintain good peak shape even when using a large injection volume. Experiments were carried out by changing the injection volume from 200 to 1800 μL. As can be seen in Figure
4, good peak separation and shape were obtained even with a sample injection of 1800 μL.
Conclusion
Simple and robust analytical and preparative SFC methods have been introduced as an alternative to the conventional HPLC analysis of OLED materials, namely TPD, methoxy TPD, and NPD. Using the same column material in both the analytical- and preparative-scale resulted in baseline separation and seamless method transfer. Switching from HPLC to SFC for the analysis of these organic compounds resulted in a significant reduction of organic solvent consumption, decreasing both the cost of analysis and the environmental impact. SFC technology offers a valuable alternative to HPLC in the analysis of OLED materials, as well as in QC.
References
- Nakajima, K., Shimadzu Corporation Application Note 01-00135-EN, 2021.
- Nakajima, K., Shimadzu Corporation Application Note 01-00136-EN, 2021.
Gesa Johanna Schad graduated with a diploma in chemical engineering from the Technical University NTA in Isny, Germany in 2004, and as a master of science in pharmaceutical analysis from the University of Strathclyde in Glasgow, UK, in 2005. She worked until 2006 as a consultant in HPLC method development and validation in an analytical laboratory of the FAO/IAEA in Vienna, Austria. She gained her doctorate for research in pharmaceutical sciences at the University of Strathclyde in 2010 and was employed as an HPLC specialist in the R&D department at Hichrom Ltd. in Reading, UK, from 2009. Since 2013, she has worked as an HPLC product specialist and since 2015 as an HPLC product manager in the analytical business unit of Shimadzu Europa in Duisburg, Germany.
Kosuke Nakajima: After earning his bachelor’s degree from Kyoto Institute of Technology in 2014 and subsequently completing his master’s degree in 2016, he began his career journey with Shimadzu in 2016. Starting as an Application Specialist, he specialized in LC, Prep LC, SFC, and MS technologies. His career path eventually took him to Shimadzu Asia Pacific in Singapore, where he assumed the role of Innovation Center Manager, leading pioneering research in state-of-the-art technologies. In 2023, he transitioned to the Shimadzu Headquarters development team, playing a role in enhancing the company’s products and technological advancements.
E-mail: info@shimadzu.eu
Website: www.shimadzu.eu

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).