Analytical Strategies in the Development of Generic Drug Products: The Role of Chromatography and Mass Spectrometry
Special Issues
A discussion of active pharmaceutical ingredient (API) selection, drug product development, and mass spectrometry instrumentation
A generic drug has the same active ingredient (or ingredients) as the reference listed drug (RLD) or branded drug (1). In many generic firms, analytical development strategies are similar to those used at branded drug firms because the analytical methods for generic drug companies must comply with the International Conference on Harmonization (ICH) and Food and Drug Administration (FDA) guidelines that also govern branded drug manufacturers (2). The different stages of generic development are active pharmaceutical ingredient (API) selection, deformulation, preformulation, and formulation development. This article will discuss API selection, drug product development, and mass spectrometry instrumentation.
Choosing an active pharmaceutical ingredient (API) supplier is a significant task in generic drug development. Figure 1 shows the different stages of generic development and how chromatography and mass spectrometry (MS) relate to each development phase (3). For sterile injectable development, the main quality attributes for an API are assay and impurity profiles. Figure 2 depicts assay and impurity profiles with respect to the International Conference on Harmonization (ICH), the Food and Drug Administration (FDA), and the European Medicines Agency (EMEA) guidelines and the separation technique commonly employed (4). One recent development in MS, which is helpful in the API selection process, is differential analysis software developed for use with liquid chromatography–mass spectrometry (LC–MS) data. Differential analysis can be used to look for very small differences in samples such as differences between two lots of APIs or differences in APIs from two different vendors. This kind of analysis is particularly important when minute differentiation among samples is required, such as in the determination of low-level impurities.
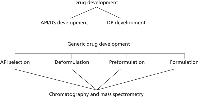
Figure 1: Different stages of generic drug product development and their relation to chromatography and mass spectrometry.
ICH Q3A addresses the quantitation and characterization of organic impurities in APIs. Quantitation of these impurities includes high performance liquid chromatography (HPLC) and ultrahigh-pressure liquid chromatography (UHPLC) methods. Characterization includes LC–MS or gas chromatography–mass spectrometry (GC–MS) analysis. ICH Q3C sets guidelines for specific residual solvents that are usually determined by GC–flame ionization detection (FID) or GC–MS. The ICH guidance document sets thresholds for reporting, identification, and quantitation as shown in Table I. When an impurity presents a probable high toxic risk or a specific structure alert to genotoxicity, new or higher levels of the impurity cannot be introduced as compared to products with existing market authorizations (1,4).
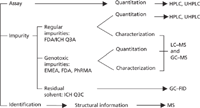
Figure 2: Assay and impurity profiles of APIs with respect to the FDA, ICH, and EMEA guidelines and separation technique commonly employed.
Drug Product Development
Chromatography and MS are extensively used in the formulation development stage of generic drug products. The ICH Q3B guidelines (Table II) address the reporting threshold, identification threshold, and qualification threshold for impurities in the drug product. In a drug product, impurities can be degradation products of the active ingredient or by-products of drug excipient incompatibility or external contaminants. Out of all of these, contaminants are the most difficult to characterize. During the development phase, chromatography and MS support drug excipient interaction studies, material compatibility studies, structure elucidation and identification studies, extractable–leachable studies, ink migration studies, and filter selection studies. Chromatography and MS also aid in unique formulation process development studies such as oxygen reduction in the headspace, bulk hold time, and lyophilization cycle development. Chromatography is a common practice for stability studies, API and drug product release, and in-process controls for the finished product.

Table I: ICH Q3A guidelines for reporting, identification, and qualification threshold limits of API
As part of an abbreviated new drug application (ANDA) filing, the impurity profile of the generic drug is compared to the reference listed drug (RLD). If an impurity is observed in the generic drug at a level in excess of the ICH Q3B guidelines, then it must be compared with the RLD to justify a higher specification. A particular impurity at the same relative retention time (RRT) observed in a branded and generic product can be deemed identical if UV spectra, MS spectra, and MS-MS spectra are the same. The exceptions to this rule are diastereomeric impurities and impurities that are structural isomers. Even though these impurities are different structurally, they may still be eluted at the same retention time. In those cases, going beyond MS-MS and carrying out a deuterium-exchange LC–MSn experiment may be helpful to differentiate them.
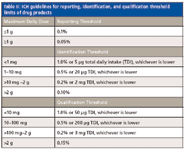
Table II: ICH guidelines for reporting, identification, and qualification threshold limits of drug products
The principal tools used for the elucidation of an unknown structure in any drug development are chromatography and MS. When a method is modified to make it compatible with LC–MS, the peaks of interest will likely have different retention times than in the original HPLC method, and may have a different elution order. Peak tracking between the release method and the adapted LC–MS method often requires that MS is run in sequence with a complementary detection method such as a diode-array detection (DAD) that can provide peak-identifying spectra for comparison between both methods.
The following is an example of how to use MS to elucidate structures of degradation products. In this example, two unknowns were observed in the stability study. These unknowns were 16 mass units higher than the API. A careful observation in the degradation chemistry revealed that the degradation product either can be through pathway A (Figure 3), where it forms hydroxide or it can be through pathway B (Figure 3), where it forms N-oxide. Hydroxide and N-oxide are identical in mass, because they are isobaric in nature. In this case, standards were not available of either N-oxide or hydroxide to compare the retention time or UV spectra or MS-MS spectra. In cases like this, deuterium-exchange chromatography combined with MS can be used to confirm structures of isobaric compounds. For the hydroxide, a deuterium-exchange chromatography–MS experiment would produce an MS peak four mass units higher than the peak for the protonated parent species. For the N-oxide, mass spectra from a deuterium-exchange chromatography–MS analysis would reveal an MS peak three mass units higher than the original protonated parent species. Figure 3 shows the deuterium-exchange mass spectra for both compounds. As observed from the mass spectra, deuterium exchange can be a powerful tool in establishing tentative structures, or may provide orthogonal proof of similarity (5). As a reminder to readers, the clinical side of the drug development process is not within the scope of this article and will not be discussed here.
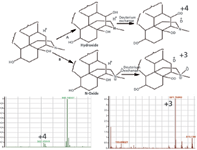
Figure 3: Structural elucidation of isobaric degradation products by deuterium-exchange LCâMS.
Recently, one issue that has been observed is that the use of columns with new column technology leads to on-column degradation, particularly in columns with particle sizes smaller than 3 µm. In Figure 4a, the on-column degradation of the drug product (DP) sample resulted in a broad, poorly defined baseline disturbance. In Figure 4b, the on-column degradation of the DP sample resulted in well defined Gaussian peaks that could easily be misinterpreted as actual impurity peaks. In the first case, the baseline disturbance is thought to be caused by degradation across the column bed. In the second case, the well-defined peaks are an indication that the degradation is occurring on the column frit and the degradation product enters the column along with the API in the narrowly defined injection plug. The degradation peaks are subsequently resolved from the active as if they were present in the analytical solution. These on-column degradation peaks were not present in brand new columns and tend to increase with the age of the column. In both cases, the resulting peaks were identified via LC–MS as oxidative degradation products of the active compound. After a tentative structure and reaction mechanism was established, a corrective process to remediate this problem was developed. It is believed that during the analysis either peroxide- or metal-mediated oxidation is taking place. The metal-mediated oxidation was controlled by flushing the column with a solution of EDTA, thereby removing the metal ions present in the column by complexation.
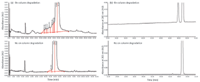
Figure 4: Example of on-column degradation peaks.
Many molecules without chromophores are often encountered in the generic drug development process. For these molecules, UV detection is not a technique of choice. So chromatography with alternate detection systems, such as refractive index (RI) detection, charged aerosol detection (CAD), evaporative light scattering detection (ELSD), reagent-free ion chromatography (RFIC), or MS can be used for developing stability indicating methods. RI detectors do not normally have enough sensitivity to detect low-level impurities. ELSD can be a powerful technique for analyzing large molecules. The utility of CAD is dependent on how well a particular molecule can maintain a charged state. Ion chromatography works best if the analytes are in ionic form. An example chromatogram of a generic drug with no chromophores analyzed with chromatography coupled with different detection systems is shown in Figure 5. In this particular case, CAD and ELSD were not able to provide enough sensitivity to identify the impurities in comparison to RFIC.
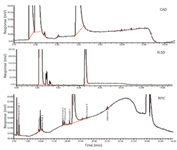
Figure 5: Chromatograms of a generic drug without chromophore using different detection systems.
MS can be a very important tool in developing fat-soluble vitamins as well, where traditional electrospray ionization (ESI) generates poorly protonated spectra. In such cases it is difficult to get protonated spectra for impurities and active ingredients. Cation-enhanced MS was shown to be very effective dealing with vitamin D derivatives (6). The sample solutions of vitamin D derivatives spiked with different molar concentrations of silver nitrate ranging from 2 mM to 6 mM were injected directly into an ESI-MS system operating in positive mode. The representative total ion chromatogram with and without silver nitrate is shown in Figure 6. The addition of silver ions drastically improved the signal corresponding to the vitamin D derivative; however, the signal response was not linear in the tested range of 2–6 mM of silver ions. The corresponding mass spectrum is shown in Figure 6. Picogram sensitivity can be achieved using this technique. The corresponding degradation products or impurities of the vitamin D derivatives can be identified using this technique.
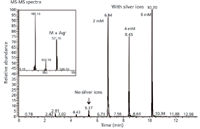
Figure 6: Cation-enhanced MS determination of vitamin D derivative with silver ions.
Mass Spectrometry Instrumentation
LC coupled with MS detection is the most advanced and powerful tool to date in the drug development process, from regular impurity identification to genotoxic impurity quantitation. The recent advances in MS have increased the selectivity and sensitivity capabilities of commercially available instruments. There are different kinds of mass spectrometers, which vary by design and ionization sources. Each instrument design has its own advantages and disadvantages. For example, triple-quadrupole mass spectrometers can provide femtomolar detection limits for certain compounds, whereas ion-trap instruments can perform multiple MSn experiments (7). However, these traditional mass spectrometers were designed to provide unit mass resolution, and hence often pose challenges for the characterization of structurally similar compounds. More recently developed instruments such as quadrupole time-of-flight (QTOF), linear orbital trap, and multiple TOF instruments provide superior mass resolution and are capable of exact mass determinations. A hybrid quadrupole–ion trap instrument provides greater specificity and quantitation and MS-MS sensitivity, thereby offering simultaneous qualitative and quantitative results (8). QTOF instruments offer enhanced selectivity, high mass accuracy of fragmented ions, and high dynamic mass range (for some instruments) (9). Linear trap combined with orbital trap instruments can do exact MS measurement with multiple accurate MS fragmentations (10). Application of these mass instruments provides quality information on elemental composition and structural characteristics with greater specificity for identifying even the least abundant compounds in complex matrices in the drug development process. With regard to ionization, newer techniques like desorption electrospray ionization (DESI) and direct analysis in real time (DART) MS promise to make the sample preparation step very simple or nonexistent (11). Improvements in ion mobility spectrometry (another form of MS) offer unique solutions for cleaning and decontamination studies.
Conclusions
Even though chromatography and MS play an integral role in any drug development, their role in generic development is even bigger because of the shorter development timelines. The future of generic analytical developments promises to be more robust with the use of newer column technology, UHPLC systems, and improvements in MS instrumentation.
Acknowledgments
The input from various colleagues from the pharmaceutical industry at large are acknowledged, particularly the members of the Product and Process Development (PPD) department at Boehringer Ingelheim (BI) Ben Venue Laboratories (Bedford, Ohio). Many helpful discussions with Mr. Greg Fernengel, Drs. Dong Wen, Richard Thompson, Robert Sullivan, and encouragement, support, and guidance provided by Dr. William Larkins are also acknowledged. The authors would also like to thank Ms. Maegan Little for proofreading the manuscript and the members of the PPD who carried out the work in the laboratory.
Disclaimer: The views provided in this articles are from authors only and not from the BI or any of its associates.
Arindam Roy and Srinivasa Gorla are with Boehringer Ingelheim Ben Venue Laboratories in Bedford, Ohio. Please direct correspondence to royarind@yahoo.com
References
(1) www.fda.gov.
(2) www.ich.org.
(3) A. Roy et al., "Analytical Strategies in the Development of Generic Drug Products: Role of Chromatography and Mass Spectrometry," presented at Pittcon 2011, Atlanta, Georgia.
(5) A. Roy et al., "Impurity Analysis in Pharmaceuticals," presented at Pittcon 2010, Orlando, Florida.
(6) G.J. Patti, Anal. Chem. 82(1), 121–128 (2010).
(7) S. Ma, Chemico-Biol. Interact. 179(1), 25–37 (2009).
(8) J.W. Hager, J. of Chrom. A 1020, 3–9 (2003).
(9) K. Imatani, Amer. Lab. 40(14) (2008).
(10) R.H. Perry, R.G. Cooks, and R.J. Noll, Mass Spectrom. Rev. 27(6), 661–699 (2008; DOI 10. 1002/mas.
(11) J.P. Williams, Rapid Commun. in Mass Spectrom. 20, 1447–1456 (2006).

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)
![Huck_Fig 2-[41825010]-{584590}_t-746305-1408610899253.gif Huck_Fig 2-[41825010]-{584590}_t-746305-1408610899253.gif](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2F0vv8moc6%2Fchroma%2Ff6261c79607ac53f41c790ed011e5b2e0d4f0626-200x140.gif%3Ffit%3Dcrop%26auto%3Dformat&w=3840&q=75)
![Roy figure 5-[41827930]-{585410}-746304-1416911904998.gif Roy figure 5-[41827930]-{585410}-746304-1416911904998.gif](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2F0vv8moc6%2Fchroma%2F5e8a7a8b42be4c1287e42a9e19c61a898804cfe5-700x592.gif%3Ffit%3Dcrop%26auto%3Dformat&w=3840&q=75)
![Schug_Fig 3-[41827280]-{641210}_t-746310-1408610889870.jpg Schug_Fig 3-[41827280]-{641210}_t-746310-1408610889870.jpg](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2F0vv8moc6%2Fchroma%2F6da0479f1e71c57976064cc832407ebe8e770158-200x104.jpg%3Ffit%3Dcrop%26auto%3Dformat&w=3840&q=75)
![Heinle Figure 1-[41825860]-{582380}_t-746308-1408610893731.gif Heinle Figure 1-[41825860]-{582380}_t-746308-1408610893731.gif](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2F0vv8moc6%2Fchroma%2F309898def0ba4426d4cd28df126a523915eb3959-200x103.gif%3Ffit%3Dcrop%26auto%3Dformat&w=3840&q=75)






