Analysis of Volatile Bacterial Metabolites by Gas Chromatography–Mass Spectrometry
LCGC North America
A method for the identification of key volatile organic compound (VOC) markers associated with infection by Neisseria meningitidis bacteria by gas chromatography–mass spectrometry (GC–MS) was developed. Headspace samples of bacterial VOCs were trapped on triple-sorbent bed tubes and then thermally desorbed into a laboratory GC–MS system for separation. Identification was carried out by comparison of GC retention time and electron ionization mass spectra to the National Institute of Standards and Technology (NIST) database. Further confirmation was obtained by GC–MS of known standard chemicals. A total of 75 VOCs were detected, five of which can be considered key VOC markers for Neisseria meningitidis. These peaks were identified as 1,2-dimethylcyclopropane, 2-methylpropanal, methacrolein, N-2-dimethyl-1-propanamine, and 3-methylbutanal by the NIST database.
A method for the identification of key volatile organic compound (VOC) markers associated with infection by Neisseria meningitidis bacteria by gas chromatography–mass spectrometry (GC–MS) was developed. Headspace samples of bacterial VOCs were trapped on triple-sorbent bed tubes and then thermally desorbed into a laboratory GC–MS system for separation. Identification was carried out by comparison of GC retention time and electron ionization mass spectra to the National Institute of Standards and Technology (NIST) database. Further confirmation was obtained by GC–MS of known standard chemicals. A total of 75 VOCs were detected, five of which can be considered key VOC markers for Neisseria meningitidis. These peaks were identified as 1,2-dimethylcyclopropane, 2-methylpropanal, methacrolein, N-2-dimethyl-1-propanamine, and 3-methylbutanal by the NIST database.

Due to the high potential for natural or intentional introduction of foreign infectious agents into the United States, tools to rapidly identify disease threats have become increasingly valuable. Recently, there has been growing interest in breath analysis. This technique is ideal for on-site analysis because it is noninvasive, rapid (compounds of interest are already in gas phase), and simpler than measurements in complex biological matrices (1–3). Breath samples from both animals and humans have been used to identify metabolic end products such as hydrogen, methane, volatile fatty acids, and other volatile organic compounds (VOCs) (4). By monitoring concentration changes of VOCs, either depleted from inhaled air (by degradation and excretion in the body) or added to exhaled breath as products of metabolism, one can determine the presence of an infection, a particular disease state, or a recent exposure to a drug or environmental pollutant (1,2,5).
To date, over 200 organic compounds have been identified in human breath (6). These compounds include hydrocarbons, alcohols, ketones, and aldehydes (1,2,7–9). The most abundant VOCs in breath are isoprene, acetone, ethanol, methanol, and other alcohols (1). Of these 200 compounds, only a few have been linked to a particular disease state. For example, breath pentane has been shown to increase in a number of diseases including breast cancer (10), heart transplant rejection (11), acute myocardial infarction (11,12), schizophrenia (11), and rheumatoid arthritis (11). Acetone has been long known to be a key metabolic byproduct in patients with diabetes (2,13) and congestive heart failure (14). Other studies have shown that an increase in concentration of dimethyl sulfide, mercaptans, and fatty acids occurs in patients with cirrhosis (2), isoprene concentration decreases in patients with heart failure (8), and dimethyl- and trimethylamine concentrations increase in patients with renal disease (2).
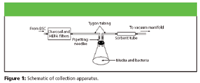
Figure 1
There have been numerous breath-analysis studies focused on the identification of disease states. However, there are surprisingly few reports in the area of bacterial infection identification. Khaled and colleagues (15) used the urea (13 C) breath test to detect Helicobacter pylori infection in humans. This same test was used to detect Helicobacter pylori infection in squirrel monkeys (16) and cats (4). Gas chromatography (GC) has proved an extremely valuable tool for identifying VOCs associated with bacterial infection in dental research (17–19). For that reason, we undertook the present study to identify key VOCs associated with infection by the Gram-negative bacteria Neisseria meningitidis (N. meningitidis) using gas chromatography–mass spectrometry (GC–MS). N. meningitidis is the major cause of both meningitis and sepsis, two diseases which have particularly high mortality rates in children and young adults (20); it infects humans exclusively (21) and is transmitted from person to person during close contact via airborne salivary droplets. The overall frequency of asymptomatic carriers is ; 10% in the general population (22), with invasive infection rates between 0.3 and 7.1 per 100,000 inhabitants in Europe (23).
Experimental
Collection apparatus: A sampling apparatus capable of collecting VOCs from the headspace gases was constructed and placed in a biological safety cabinet (BSC). A vacuum pump (Gast Manufacturing, Benton Harbor, Michigan) was connected to a six-port stainless steel manifold with 0.25-in. perfluoroalkoxy (PFA) tubing (Curbell Plastics, Orchard Park, New York). Each sampling port had a critical orifice (O'Keefe Controls Co., Monroe, Connecticut) that allowed a constant flow rate of 62 mL/min when a vacuum draw greater than 15 in. Hg was applied. The stainless steel sorbent tubes (Markes International, Pontyclun, UK) were 0.25-in. o.d. X 3.5- in. long and attached to the manifold ports via Swagelok fittings (Solon, OH) using Teflon ferrules. The 150-cm2 polystyrene cell culture flasks (Corning, Corning, New York) were sealed with silicone stoppers (Fisher Scientific, Pittsburgh, Pennsylvania) during sampling to limit the loss of headspace gases and to minimize VOC off-gassing. Gas flow into and out of the culture flasks during collection occurred through pipetting needles that penetrated the stoppers. Headspace collection was completed by connecting a sorbent tube to the tissue culture flask through autoclaved Tygon tubing connected to a 6-in. 14-gauge pipetting needle (Fisher Scientific, Pittsburgh, Pennsylvania). The evacuated headspace gas was replaced with outside air that was charcoal and high-efficiency particulate air (HEPA) filtered (Fisher Scientific, Pittsburgh, Pennsylvania) from the biological safety cabinet through a 2-in. 16-gauge pipetting needle. The headspace gases were drawn through sorbent traps designed to retain volatile organic compounds.

Figure 2
The sorbent tubes that were employed use a "triple sorbent" bed approach, in which three sorbent materials with increasing retentive capabilities for VOCs are packed in series such that easily retained volatile compounds are trapped in the first sorbent bed while the more volatile and the more difficult to trap compounds that breech the early sorbent beds of the tube are subjected to increasingly retentive materials. The triple sorbent bed contained Carboxen 1000, which traps carbon chain lengths of C2–C5; Carbotrap B, which traps carbon chain lengths of C5–C12; and Carbotrap C, which traps carbon chain lengths of C12–C20. The design of the sampling system allows for easy removal and replacement of both sorbent tubes and tissue culture flasks. The system is capable of sampling VOCs from as much as six samples simultaneously, and is of a size such that the entire system could fit readily into an incubator or biological safety cabinet.

Table I
Bacterial strain, cultivation, and collection of headspace samples: Headspace samples of bacterial VOCs from N. meningitidis (ATCC # 13077 type strain, isolated from spinal fluid from a patient with fatal meningitis) were obtained from CUBRC (Buffalo, New York) and handled under biosafety level-2 (moderate infection risk) guidelines. The N. meningitidis bacteria were cultured in Roswell Park growth media (RPMI-1640) with murine macrophages (ATCC # CRL-2019) for varying amounts of time (1, 3, 6, 24, 48, and 72 h). Two samples were collected at each interval along with a background sample of ambient room air. Empty sorbent tubes, media-only cultures, and media/cell cultures were used as control runs.
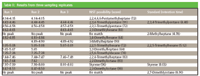
Table II
Equipment and procedure: Volatile organic compounds were collected on a mixed bed stainless steel sorbent tube (3.5 in. X 0.25 in. o.d.) packed with Carboxen 1000/ Carbopack B/Carbopack C. The tubes were loaded into a Markes Ultra TD automated sampler (capacity 100, Pontyclun, UK), which was interfaced to a Markes Unity Thermal Desorber (Pontyclun, UK). The tubes were checked for leaks, and purged with helium for 1.0 min to remove water and air. The tube was then desorbed at 280 °C for 5 min onto a -10 °C Tenax cold trap (with a flow rate of 10 mL/min). The cold trap was then heated rapidly to 280 °C and the desorbed sample was flushed through a fused silica transfer line (0.25-mm i.d., 180 °C) to the chromatographic column (30 m X 0.25 mm, 0.5-μm df Rtx-5Sil MS, Restek, Bellefonte, Pennsylvania). Separation via GC was achieved by ramping the column temperature as follows: 40 °C, hold 1 min, 6 °C/min to 280 °C, hold 10 min. The total analysis time was 51.0 min. The GC–MS system employed in this study was a Hewlett Packard (HP) Model 6890 Gas Chromatograph (Palo Alto, California) interfaced to a HP model 5973 Mass Selective detector with ChemStation software for data acquisition and processing.

Table III
Total ion current was monitored for all samples using electron-impact ionization (70 eV) in the positive ion mode. A scan rate of 2.0 scan/s and mass range of 20–500 amu was applied. Data from each chromatogram, including peak retention time, area under the curve, and tentative identification based upon matching of mass spectral database, were transferred to an Excel spreadsheet. Tentative identification of the chemical structure of these compounds was based upon interpretation of the mass spectra (fragmentation patterns and ion abundance) and library matching using the NIST/EPA/NIH mass spectral database (Gaithersburg, Maryland, Version 2.0). Further confirmation of potential marking compounds was conducted by purchasing reference standards of the tentatively identified species and performing GC–MS analysis using the same thermal desorption and chromatographic conditions employed for the headspace samples.
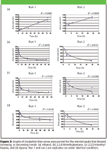
Figure 3
Data management: Cell cultures were incubated for intervals of 1, 3, 6, 24, or 72 h and repeated in triplicate. The cell culture contained media only, media with cells, or media with cells and bacteria. The GC run lasted 51 min, although no significant peaks were eluted after 18 min. A typical GC chromatogram is shown in Figure 2. The retention time, area percent (or corrected area), and database search results were exported into Excel spreadsheets. The spreadsheets were used to compare peak areas of eluted VOCs between blank, media-only, media with cells, media with cells and bacteria, incubation time, and day of experiment. The eluted VOCs fell into one of six categories: peak areas that increased over incubation time, peak areas that decreased over incubation time, peak areas that increased at first then decreased, peak areas that decreased then increased, peak areas that remained relatively constant, or peaks which only appeared when incubated with bacteria. To further clarify which peak areas showed changes, graphs of incubation time versus area percent (or corrected area) were made. Representative graphs of selected peaks are shown in Figure 3.
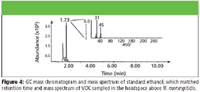
Figure 4
The NIST search results were used to select standards needed to identify of peaks of interest. In all, eight standards (ethanol, dimethyl sulfide, 1,3,5-trifluorobenzene, 2,3,4-trimethylpentane, 2-methylheptane, 2,2,5-trimethylhexane, styrene, and 2,7-dimethyloctane) were selected and analyzed. Each standard was injected manually and run under the same GC–MS conditions as the cell culture headspace samples.
Figure 5
Results and Discussion
A total of 75 VOCs were detected in the headspace of N. meningitidis cultures. Six of these compounds were identified by comparison to standards: ethanol, dimethyl sulfide, 1,3,5-trifluorobenzene, 2,3,4-trimethylpentane, 2,2,5-trimethylhexane, and styrene (Figures 4–9). A typical GC chromatogram contains most (but not all) of the 75 VOCs. Ethanol, 2,3,4-trimethylpentane, 2,2,5-trimethylhexane, and styrene were detected over all three days of sampling in all replicates. Dimethyl sulfide and 1,3,5-trifluorobenzene were detected only in one of the three replicates. Five of these compounds previously have been identified in human breath: 2,3,4-trimethylpentane (11), 2,2,5-trimethylhexane (12), dimethyl sulfide (24), ethanol (24), and styrene (11). Styrene also has been detected in cattle breath (4).
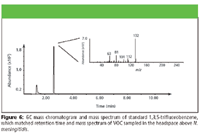
Figure 6
Two other standards, 2-methylheptane and 2,7-dimethyloctane, were analyzed but did not match any of the candidate peaks. However, the mass spectrum of 2,7-dimethyloctane (Figure 10) was close to matching the candidate mass spectrum found in the GC chromatogram (Figure 11). Therefore, it is believed that the structure of the candidate peak is similar to 2,7-dimethyloctane, and it might be a related isomer.
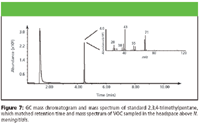
Figure 7
Tables I–III list the results obtained from each replicate. Incubation and collection of headspace were conducted in the same manner for all three replicates. Each sampling replicate consisted of collection times at 1, 3, 6, 24, or 72 h of incubation. Only the retention times of peaks resulting in an NIST hit are shown, along with the NIST result. The standards used along with the corresponding retention time are also shown. Of the 25 peaks listed, seven were found to increase with incubation time (Table I; retention times 1.6, 1.71, 1.94, 2.02, 2.41, 2.64, and 3.14 min). Only ethanol (retention time 1.71 min) was found to increase in all three runs, indicating that it is a metabolic product (as indicated also in Figure 2a). The peaks at 1.94 and 2.64 min were identified as dimethysulfide and 1,3,5 trifluorobenzene, respectively. Sixteen peaks were found to decrease over the 72-h incubation time (Table II). Five of these peaks (retention times 4.43, 4.56, 5.05, 7.44, and 7.97 min) were detected in all three replicates. The peaks found at 4.43, 5.05, and 7.97 min were identified as 2,3,4-trimethylpentane, 2,2,5-trimethyhexane, and styrene, respectively (as indicated also in Figures 2b–d). Seven peaks (retention times 4.14, 4.62, 4.82, 4.89, 7.35, 7.61, and 8.47 min) were detected in two of the three replicates. Combined, there were only five peaks that appeared after bacteria were added to the culture (Table III). The peak at 1.93 min was the only peak detected in more than one replicate. From our analysis, it was determined that the majority of peaks that increased occurred before 3 min elution time; the peaks that decreased eluted between 4 and 10 min after injection.
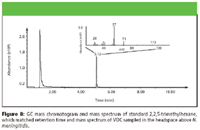
Figure 8
As a comparison, the bacteria were analyzed without media or cells. This resulted in 16 peaks that were present with cells and media runs only. However, a total of 59 peaks were detected when media and cells were added to the bacteria. The N. meningitidis bacteria produced five unique peaks occurring at retention times 1.93, 2.15, 2.22, 2.56, and 3.02 min. These peaks were identified by the NIST database as 1,2-dimethylcyclopropane, 2-methylpropanal, methacrolein, N-2-dimethyl-1-propanamine, and 3-methylbutanal, respectively.

Figure 9
Conclusions
This study shows that N. meningitidis bacteria produce VOCs that can be detected by GC–MS. A total of 75 VOCs were detected, five of which can be considered key VOC markers for Neisseria meningitidis. These peaks were identified as 1,2 dimethylcyclopropane, 2-methylpropanal, methacrolein, N-2 dimethyl-1-propanamine, and 3-methylbutanal by the NIST database. As expected, many of the VOCs detected were hydrocarbons, alcohols, ketones, and aldehydes. Further studies must be performed on additional samples to establish whether VOC biomarkers of infection for different bacteria can be determined, as well as diagnosis of infection in animal and human models.
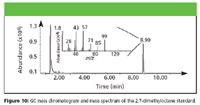
Figure 10
Acknowledgments
The authors would like to thank the Armed Forces Medical Intelligence Center (AFMIC) for financial support.
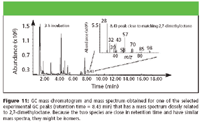
Figure 11
William L. Wood*, Daniel J. Higbee*, Melanie Gooldy†, Scott Glogowski†, Rich Fitzpatrick†, Richard J. Karalus†, Troy D. Wood*, David J. Mangino†
*Department of Chemistry
University at Buffalo, The State University of New York
Natural Sciences Complex
Buffalo, New York
†CUBRC
Buffalo, New York
Please direct correspondence to Troy D. Wood at twood@buffalo.edu
References
(1) J.M. Sanchez and R.D. Sacks, Anal. Chem. 75, 2231–2236 (2003).
(2) A. Manolis, Clin. Chem. 29, 5–15 (1983).
(3) K.M. Dubowski, Clin. Chem. 20, 966–972 (1974).
(4) J.P. Spinhirne, J.A. Koziel, and N.K. Chirase, J. Chromatogr., A 1025, 63–69 (2004).
(5) J.D. Pleil and A.B. Lindstrom AB, Clin. Chem. 43, 723–730 (1997).
(6) L. Pauling, A.B. Robinson, R. Teranishi, and P. Cary, Proc. Nat. Acad. Sci. USA 68, 2374–2376 (1971).
(7) T. Qin, X. Xu, T. Polak, V. Pacakova, K. Stulik, and L.A. Jech, Talanta44, 1683–1690 (1997).
(8) L.T. McGrath, R. Patrick, and B. Silke, Eur. J. Heart Failure 3, 423–427 (2001).
(9) M. Phillips, Anal. Biochem. 247, 272–278 (1997).
(10) E. Hietanen, H. Bartsch, J.C.Bereziat, A.M. Camus, S. McClinton, O. Eremin, L. Davidson, and P. Boyle, Eur. J. Clin. Nutrition 48, 575–586 (1994).
(11) M. Phillips, J. Herrera, S. Krishnan, M. Zain, J. Greenberg, and R.N. Cataneo, J. Chromatogr., A 729, 75–88 (1999).
(12) Z.W. Weitz, A. J. Birnbaum, P.A. Sobotka, E.J. Zarling, and J.L. Skosey, Lancet 337, 933–935 (1991).
(13) C. Deng, J. Zhang, X. Yu, W. Zhang, and X. Zhang, J. Chromatogr., B 810, 269–275 (2004).
(14) M. Kupari, J. Lommi, M. Ventila, and U. Karjalainen, Amer. J. Cardiol. 76, 1076–1078 (1995).
(15) M.A. Khaled and S.A. Sarker, Nutrition Res. 18, 1463–1468 (1998).
(16) C.T.K.H. Sadtlander and F.J. Stutzenberger, Lab. Animal Sci. 45, 239–242 (1995).
(17) M.V. Stack, J. Chromatogr. 165, 103 –116 (1979).
(18) S. Persson, M.-B. Edlund, R. Claesson, and J. Carlsson, Oral Microbiol. Immunol. 5, 195–201 (1990).
(19) S. Persson, Oral. Microbiol. Immunol. 7, 378–379 (1992).
(20) D. Serruto, J. Adu-Bobie, B. Capecchi, R. Rappuoli, M. Pizza, and V. Masignani J. Biotechnol. 113, 15–32 (2004).
(21) M.-K. Taha, A.-E. Deghmane, A. Antignac, M.L. Zarantonelli, M. Larribe, and J.-M. Alonso, Trends Microbiol. 10, 376–382 (2002).
(22) K. Cartwright, "Meningococcal Carriage and Disease in Meningococcal Disease," K. Cartwright, Ed. (John Wiley & Sons, Chichester, UK,1995), pp. 115–146.
(23) N. Noah and B. Henderson, in Surveillance of Bacterial Meningitis in Europe 1999–2000 (PHLS, London, UK, 2001).
(24) W. Miekisch, J.K. Schubert, and G.F.E. Noeldge-Schomburg GFE, Clin. Chim. Acta 347, 25–39 (2004).

Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.
Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.












