Amide Hydrogen–Deuterium Exchange: A Fast Tool for Screening Protein Stabilities in Chromatography
LCGC North America
Protein unfolding and aggregation can be serious considerations when designing laboratory and preparative chromatographic purification steps. This problem has been studied most thoroughly within the contexts of reversed-phase chromatography and hydrophobic interaction chromatography. However, there are currently no robust methods for resin selection capable of predicting adsorbed-phase protein stability as a function of amino acid sequence, secondary or tertiary structure, or resin characteristics.
Protein unfolding and aggregation can be serious considerations when designing laboratory and preparative chromatographic purification steps. This problem has been studied most thoroughly within the contexts of reversed-phase chromatography and hydrophobic interaction chromatography. However, there are currently no robust methods for resin selection capable of predicting adsorbed-phase protein stability as a function of amino acid sequence, secondary or tertiary structure, or resin characteristics. Conventional experimental techniques such as circular dichroism and intrinsic fluorescence cannot be applied easily to adsorbed protein on conventional chromatographic matrices because of the opacity of the base matrix. Amide hydrogen–deuterium exchange is a protein-labeling technique sensitive to changes in tertiary structure that has been employed extensively to study conformational changes in a variety of protein-folding studies in solution. Here, we present a methodology for using amide hydrogen–deuterium exchange with chromatographic materials as a means to identify buffer and resin conditions that are destabilizing to specific proteins. The approach is illustrated with hen egg white lysozyme and bovine α-lactalbumin adsorbed to Phenyl Sepharose 6 FF high substitution resin under strong binding conditions. Labeling patterns directly demonstrates the strong differences in stability during binding that can lead to irreversible adsorption or low recoveries.

Protein unfolding and aggregation during chromatographic purification steps often complicate process development efforts and compromise product yields. The presence of secondary equilibria between native and denatured protein conformations also has a dramatic effect on retention and peak shape in chromatographic processes, as has been shown for reversed-phase chromatography (1) and displacement chromatography (2). Experimental studies have demonstrated conclusively that a number of proteins undergo surface-induced conformational changes during reversed-phase chromatography and that unfolding in these cases can be quite severe (3–5). Moreover, it has been shown that the amount of unfolding on reversed-phase columns can be influenced by stationary phase characteristics such as carbon loading and pore size (6) and operating conditions such as protein loading (7). Adsorption on hydrophobic interaction chromatography (HIC) surfaces also have been shown to denature the moderately stable protein bovine α-lactalbumin (BLA) (8,9). The kinetics of BLA unfolding on both strongly hydrophobic polystyrene reversed-phase chromatography-like (10) and weakly hydrophobic HIC (9) surfaces has been investigated with intrinsic fluorescence and was found to be biphasic in both cases. The rates of unfolding were dramatically higher on the more hydrophobic surface. Biphasic unfolding kinetics indicates the presence of three conformations of adsorbed protein: native, fully unfolded, and a partially unfolded intermediate state. Clearly, the nature and severity of adsorption-induced protein unfolding is dependent upon many variables, including biophysical properties of the protein molecule itself.
The advent of recombinant DNA technology and mammalian cell culture have placed special emphasis on the need for purification techniques amenable to large globular, glycosylated proteins, especially monoclonal antibodies. Currently, aggregation of these molecules during low-pH elution steps from protein A columns is a large problem, and selecting the best operating conditions and additives can be very time-consuming (11). Ion-exchange chromatography also has been implicated in the formation of soluble antibody aggregates at low-pH conditions (12). Identifying conditions that stabilize newly engineered, novel proteins during chromatographic purification can be especially difficult because the solution-phase structure and folding behavior of these molecules might not be known.
We have developed a batch chromatography experiment that employs amide hydrogen–deuterium exchange (HX) labeling to quickly identify protein conformational changes during adsorption with a minimum of resin and sample consumption. Unlike many spectroscopic methods, HX labeling is highly sensitive to changes in protein tertiary structure and is well suited to use with adsorbed protein. Interpretation of the experimental results is relatively straightforward — a bimodal distribution of peak areas in a single mass spectrum is representative of the number of protein molecules that underwent conformational change during the labeling period while adsorbed to the resin. Mass spectra obtained following labeling with a number of different stationary phases, buffers, and additives can be compared to determine which set of operating conditions is most stabilizing to the protein being purified.
HX labeling is initiated by diluting a concentrated protein solution into a buffer containing D2O rather than H2O. Each amide hydrogen atom (covalently bonded to the nitrogen atom in a peptide bond) has the ability to exchange with a deuteron at a rate that depends strongly upon solvent accessibility and involvement in hydrogen bonding (13). For globular proteins, the solvent accessibility of a given amide hydrogen atom is largely determined by the nature of the polypeptide's three-dimensional (3-D) fold and how deeply the amide group is buried within the hydrophobic core of the molecule (14). For a buried amide hydrogen atom, exchange is dependent upon protein unfolding, as described by the two-step model (13):
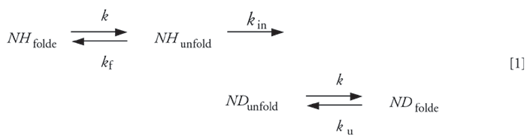
where NH and ND represent protonated and deuterated amide groups, respectively, and ku, kf, and kintr are the rate constants for protein unfolding, protein folding, and intrinsic hydrogen–deuterium exchange, respectively. The intrinsic exchange rate is the rate that would be observed for an amide hydrogen atom that was completely solvent-exposed. Completely solvent-exposed amides are found at the surface of globular proteins or in an unstructured peptide. The hydrogen exchange reaction is catalyzed by D3O+, OD-, and D2O; therefore, it is a strong function of pD (15–17):
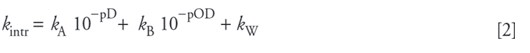
Here, kA, kB, and kW are the acid-catalyzed, base-catalyzed, and water-catalyzed rate constants, respectively. kA, kB, and kW are functions of temperature and ionic strength and are also affected by steric and inductive effects from neighboring amino acid sidechains. Correction factors for the side chain effects and activation energies for the acid- and base-catalyzed reactions (necessary for temperature correction) have been estimated previously and are available in the literature (18,19). These studies also provide estimates for kA, kB, and kW at various conditions.
Hydrogen exchange is primarily base-catalyzed at near-physiologic (and higher) pH values. Under these conditions, if the effects of amino acid nearest neighbors are neglected, the intrinsic exchange rate can be estimated by (18):

(kB is approximately 1.12 X 1010 M-1 min-1 at 20 °C and low ionic strength [18]). The molar ionization constant of D2O (KC,D2O) is different than that of H2O; at 20 °C, KC,D2O is approximately 10–15.05 (20). Assuming ideal solution conditions, the relationship between KC, D2O, and pD and pOD is analogous to that for water (20):

Also, pH meters that have been calibrated with H2O-based buffers give an artificially low pD reading when used with D2O-based solutions. A correction factor of 0.4 pH units can be applied across the entire pH range (21):

where pHread is the actual meter reading in a deuterated solution. For a protein solution in D2O, the pD is 7.4 if the measured pH value is 7.0. At 20 °C, the pOD of this solution is 7.65 (equation 4). From equation 3, the intrinsic rate for hydrogen exchange at these conditions is 250.7 min-1 . At 20 °C and low salt conditions, the overall intrinsic exchange rate (equation 2) reaches a minimum value of approximately 0.1 min-1 at pH 3 (18,22) (neglecting amino acid side chain effects).
It is important to note that other functional groups found in amino acid sidechains (such as hydroxyl, carboxyl, thiol, amino, and imido) also are capable of hydrogen exchange. Hydrogen atoms in these groups also potentially could serve as conformationally sensitive reporter groups. However, the intrinsic exchange rates of these groups are orders of magnitude higher than amide groups (22) at all pH values. If hydrogen-exchange labeled protein samples are desalted or otherwise processed on reversed-phase media before analysis, these groups can be expected to exchange back to hydrogen if H2O-based reversed-phase solvents are used. Typically, sample processing with reversed-phase chromatography involves low-pH buffers, a highly hydrophobic surface, and organic solvents, all of which favor protein unfolding and solvent exposure of the entire polypeptide backbone. An alternative approach involves the use of deuterated solvents for reversed-phase chromatography sample processing, in which case all of the sidechain functional groups would be exchanged before sample analysis. In either case, pH control during sample processing is crucial to maintaining the integrity of the isotopic label if analysis conditions are harsh enough to cause protein unfolding.
Amide HX labeling patterns have been analyzed in three ways: two-dimensional nuclear magnetic resonance imaging (2-D NMR) (5,6,14,23,24), online high performance liquid chromatography–mass spectrometry (HPLC–MS) (25–36), and matrix-assisted laser-desorption ionization–mass spectrometry (MALDI–MS) (37,38). Analysis with 2-D NMR allows the highest possible spatial resolution of differences in solvent exposure, as each amide group gives rise to a separate cross-peak in a 2-D NMR spectrum. This means that every residue potentially can serve as a reporter group, except proline, which contains no amide hydrogen atom. The disadvantages of NMR are longer analysis times and greater sample consumption than HPLC–MS or MALDI–MS. Also, NMR is not suitable for analysis of larger proteins such as antibodies (23). In HPLC–MS, intact proteins or peptides typically are concentrated and desalted on a reversed-phase chromatography column, and then are eluted directly into the mass spectrometer using an acetonitrile:water gradient. Switching valves normally are used to divert salts away from the MS electrospray ionization (ESI) source, which uses a combination of high voltage, high temperature, and an inert nebulizing gas to vaporize protein molecules. The upper limit on the size of protein molecules that can be ionized with ESI is large. However, commonly available mass spectrometers equipped with ion trap type analyzers typically suffer from poor signal-to-noise ratio when scanning higher molecular weight proteins above 50 kDa. Mass spectrometers with time-of-flight (TOF) analyzers are well suited to the analysis of large proteins such as antibodies; even intact viruses (39) and a ribosome (40) have been analyzed with this method. For HX–MS studies, post-translational modification of protein molecules (such as glycosylation) can complicate MS analysis because of the resulting heterogeneity in the molecular weight distribution. Both size limitations and post-translational modification issues can be overcome with peptide-level HX–MS. In that case, spatial resolution of labeling patterns is improved by enzymatically digesting the molecule with an acid-active protease (usually porcine pepsin) under low-pH and low-temperature conditions. Proteolytic digestion can be accomplished by adding soluble pepsin to the sample bolus (25,28,31,33,34) or by adding an immobilized pepsin column to the online HPLC–MS apparatus (26,27,29). HPLC–MS analysis of intact proteins yields only "global" information about the overall solvent exposure of molecules. However, this can be a powerful approach because subpopulations of molecules with different conformations or folding histories will give rise to separate peaks in a mass spectrum following HX labeling. This allows the distribution of conformations to be analyzed. Conversely, NMR measurements of hydrogen exchange provide only the degree of labeling averaged over all molecules in a sample. Hydrogen exchange detected by MS has been used successfully to study aggregation of a pharmaceutical protein (32), investigate selectivity differences on HIC resins (35), and identify transient intermediates in protein-folding pathways (36). Intact, HX-labeled proteins also can be analyzed with MALDI–MS, a form of MS that is more tolerant of nonvolatile salts and does not require the use of reversed-phase columns for desalting. To date, this technology has been used with hydrogen exchange primarily for the purpose of high-throughput stability determination of proteins in unpurified samples (38).
Here, we present illustrative results using hydrogen exchange HPLC–MS to analyze the relative stability of two model proteins, bovine α-lactalbumin and hen egg white lysozyme, while adsorbed to a commercial HIC resin. The results reveal which of these two structurally similar proteins is more stable on the resin: bovine α-lactalbumin is highly destabilized in the adsorbed phase, a result that could not have been predicted easily based upon amino acid sequence or tertiary structure.
Experimental
HIC resin slurry preparation: Phenyl Sepharose 6 Fast Flow high substitution media (GE Healthcare, Piscataway, New Jersey) was exchanged into the working ammonium sulfate buffer (1.5 M ammonium sulfate, 12 mM calcium chloride or 2 mM EDTA, 25 or 45 mM phosphate, pH 7.0) by stepwise dilution. A 1-mL volume of HIC resin in 20% ethanol storage solution was added to 9 mL of ammonium sulfate buffer in a graduated polyethylene centrifuge tube. The resin was allowed to settle completely, and all liquid was removed with a pipet. Fresh ammonium sulfate buffer was added to the settled resin for a final volume of 10 mL. After settling, this process was repeated a second time. Finally, fresh ammonium sulfate buffer was added to give a final slurry-to-settled bed ratio of 2:1 by volume.
Batch adsorption and hydrogen-deuterium exchange: A 200-μL volume of the HIC resin slurry prepared as described previously and 100 μL of protein solution (20 mg/mL in working ammonium sulfate buffer) were added to a 5-mL glass vial at ambient temperature. The mixture was stirred for 30 min at low speed with a magnetic micro-stir bar. Hydrogen exchange labeling was initiated by adding 1800 μL of deuterium-based ammonium sulfate buffer. The final deuterium content of the exchangeable pool of hydrogen and deuterium was 76.6 (mole)%, accounting for the hydrogen donated by ammonium sulfate. Labeling was quenched after 10 min by adding 200 μL of water-based quench buffer (1.5 M ammonium sulfate, 12 mM calcium chloride or 2 mM EDTA, 600 mM phosphate, pH 1.5), giving a final sample pD of approximately 3.0. A 500-μL volume of the quenched sample was pipeted into a 0.22-μm Amicon centrifugal filter (Millipore, Billerica, Massachusetts) and centrifuged at 7.5 rcf for 1 min to remove supernatant. The filter unit was then removed and transferred to a clean centrifuge tube. A 200-μL volume of elution buffer (25 mM phosphate pD 3.0, 78.3 (mole)% D2O, 21.7 [mole]% H2O) was added to the resin. The sample was spun again at 7.5 rcf for 1 min, and the entire volume of filtrate was processed immediately via HPLC–MS.
Protein concentration in the supernatant phase (Csup) was determined by UV absorbance at 280 nm. Immediately before quenching, a 500-μL aliquot of labeled sample was removed and spun down in a centrifugal filter unit (described previously) to remove resin. The supernatant was analyzed in a model DU 640 Series UV spectrophotometer with a 1-cm path length cell (Beckman-Coulter, Fullerton, California). The adsorbed protein concentration based upon the settled resin volume (q) was calculated by mass balance as follows:

Protein recovery was determined by stripping the resin with 8 M guanidine hydrochloride. Following elution, 200 μL of guanidine hydrochloride solution was added to the filter unit containing the resin. After approximately 15 min, the filter unit was transferred to a clean centrifuge tube, and the sample was centrifuged at 7.5 rcf for 1 min. Protein concentration in the filtrate (Cstrip) was determined by UV absorbance at 280 nm, and recovery (R) was calculated as:
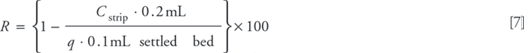
HPLC–MS
Samples were injected into a 200-μL stainless steel sample loop using a 500-μL glass syringe (Hamilton, Reno, Nevada). The stainless steel sample loop was mounted on a two-way, six-port injection valve (Rheodyne, Rohnert Park, California). Labeled protein molecules were concentrated and desalted on a 12.5 mm X 2.1 mm Zorbax C3 guard column (Agilent Technologies, Wilmington, Delaware) for 2 min at 0.5 mL/min. Following desalting, protein was eluted from the reversed-phase column with a 5-min gradient from 5 to 80% solvent B. Solvent A was ddH2O with 0.05% trifluoroacetic acid, and solvent B was acetonitrile with 0.04% trifluoroacetic acid. HPLC solvents were degassed on-line and delivered by a SpectraSystem P4000 gradient pump (Thermo Electron Corporation, San Jose, California). The sample loop, injection valve, reversed-phase column, and solvent inlet line were submerged in an ice-water bath to minimize back-exchange.
Protein that was eluted from the reversed-phase column was pumped directly to the ESI source of an LCQ Duo mass spectrometer (Thermo Electron). Flow was split from 0.5 mL/min to approximately 30 μL/min immediately before the ESI source. The ESI source capillary temperature was 200 °C and the spray voltage was 4.2 kV. Mass spectral data were collected in single-ion monitoring (SIM) mode from m/z 1770 to 1790 for α-lactalbumin or m/z 1785 to 1805 for hen egg white lysozyme. Data for the +8 charge state of α-lactalbumin and lysozyme were deconvoluted manually in Excel (Microsoft, Redmond, Washington).
No surface control experiments: Control experiments in the absence of the HIC surface were initiated by diluting 300 μL of protein solution (2 mg/mL α-lactalbumin or 0.5 mg/mL lysozyme in water-based ammonium sulfate buffer) into 1800 μL of deuterium-based ammonium sulfate buffer. The final deuterium content was 76.6 (mole)%, accounting for hydrogen donated by ammonium sulfate. The mixture was incubated at ambient temperature. After 10 min, a 500-μL sample aliquot was removed and quenched with 50 μL of water-based quench buffer (1.5 M ammonium sulfate, 12 mM calcium chloride or 2 mM EDTA, 600 mM phosphate, pH 1.5), giving a final sample pD of approximately 3.0. Quenched samples were processed immediately on the HPLC–MS system as described previously.
Fully labeled controls: Control experiments to determine the maximum possible degree of labeling also were performed. Complete labeling of α-lactalbumin (0.05 mg/mL) was accomplished by incubation of the protein in deuterium with 8 M deuterated guanidine{DCl at ambient temperature for 24 h. The ratio of guanidine{DCl to guanidine·HCl was such that the final deuterium content was approximately 76.6 (mole)%, accounting for the protons donated by guanidine. Following labeling, 500 μL of sample was removed and quenched with 50 μL of 1.0% glacial acetic acid in deionized water to give a final pD of 3.0. The final solution was immediately processed on the HPLC–MS system as described previously.
Complete labeling of hen egg white lysozyme (0.05 mg/mL) was accomplished by incubation of the protein in deuterium with 5.4 M guanidine·HCl at ambient temperature for 24 h. The final deuterium content was approximately 77 (mole)%, accounting for the protons donated by guanidine hydrochloride, but not accounting for volume changes upon addition of salt. Following labeling, 500 μL of sample was removed and quenched with 50 μL of 1.0% glacial acetic acid in deionized water to give a final pD of 3.0. The final solution was immediately processed on the HPLC–MS system as described previously.
The loss of isotopic label during sample processing, or back-exchange (B), was calculated from the fully labeled control experiments using the formula:
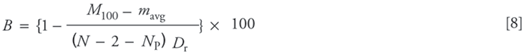
where M100 is the experimental molecular weight from the fully labeled control experiment, and mavg, N, and NP are the average mass of unlabeled protein, the number of residues in the protein sequence, and the number of proline residues in the sequence, respectively. Dr is the mole fraction of deuterons in the pool of protons (H) and deuterons (D) available for exchange in solution (0.766 in this case). Proline residues are not counted because they do not have amide hydrogens. The first residue in the sequence is discounted because it does not have a peptide bond or an amide group. The second residue is discounted because an inductive effect from the positively charged N-terminus causes the intrinsic exchange rate of this amide to be unusually high (18). Back-exchange losses in these experiments were 16% for α-lactalbumin and 15% for lysozyme.
Materials: Type I bovine α-lactalbumin and hen egg white lysozyme were purchased from Sigma-Aldrich (St. Louis, Missouri). Deuterium oxide was purchased from Cambridge Isotope Laboratories, Inc. (Andover, Massachusetts).
Results
The observed HX labeling patterns show that adsorption to a HIC surface can have profoundly different effects on the tertiary structure of different proteins (Figure 1). The overall solvent accessibility of lysozyme was very similar when labeled in solution and while adsorbed to Phenyl Sepharose 6 FF high substitution media. This is indicated by the nearly identical positions of the surface-labeled peak and the no-surface control peak in the mass spectrum (two mass spectra shown in Figure 1a). The first moment of the surface-labeled lysozyme peak is located at 14,364 Da, and the first moment of the solution-phase control is at 14,365 Da. While it is somewhat unexpected that adsorbed-phase protein would display greater protection against hydrogen exchange than the solution-phase protein, the small difference between these values is well within the instrumental error of the mass spectrometer (about 2–3 Da for a small protein). Both of these values are significantly less than the fully labeled mass of the protein (vertical dashed line in Figure 1a). The lack of signal intensity at the fully labeled value indicates that an insignificant fraction of protein molecules were unfolded during the labeling period. Bovine α-lactalbumin, in contrast, displays very different behavior in the adsorbed phase; a distinct second peak is present in the mass spectra at both buffer conditions studied (Figures 1b and 1c). The existence of a second, heavier peak in the resin-phase mass spectrum but not in the solution-phase control spectrum means that some lactalbumin molecules became more solvent-exposed while adsorbed to Phenyl Sepharose 6 FF high substitution resin. An increase in solvent exposure is indirect evidence that the tertiary structure of lactalbumin is significantly perturbed by adsorption to this resin.
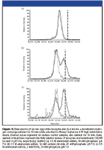
Figure 1
Bovine α-lactalbumin, a metal-binding protein, is stabilized by the presence of calcium ions (41). At neutral pH, in an ammonium sulfate buffer, and at ambient temperature, the stabilizing effect of calcium on lactalbumin is not readily apparent from hydrogen-exchange labeling in solution; the no surface control spectra both have one peak (Figures 1b and 1c). In the presence of 12 mM calcium chloride, the lactalbumin control spectrum has a peak at 14,228 Da, and in 2 mM EDTA, the peak is at 14,227. The difference between these values is less than the instrumental error. The heavy peaks in the surface-labeled mass spectra also appear in, effectively, the same positions (14,251 Da in the presence of 12 mM calcium chloride, 14,254 Da in the presence of 2 mM EDTA); however, the ratio of heavy-to-light peak is clearly higher when calcium is not available to lactalbumin. Lysozyme, more stable than both calcium-bound and calcium-depleted lactalbumin (see Table I), does not display any heavy peak whatsoever. For the case of lactalbumin and lysozyme, amide hydrogen–deuterium exchange has elucidated a rough correlation between protein stability and the amount of unfolding observed on the HIC resin.
Incomplete recovery of all the protein from the resin makes quantitation of overall protein unfolding difficult, but it does not cloud the major trends observed in the mass spectra. Approximately 93% of the lysozyme and 80% of the lactalbumin bound to Phenyl Sepharose was recovered – this was the large majority of protein in all cases (see Table I). Thus, the exchange-labeled mass spectra are representative of the protein that was removed from the resin under typical elution conditions. We cannot speak to the conformational fate of the small fraction of protein that was not recovered; however, it is likely that this protein was unfolded or aggregated on the resin. In this case, it appears that unfolding on the resin can be responsible for increased yield losses of lactalbumin relative to lysozyme.
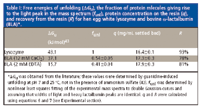
Table 1
These results also shed some light on the nature of the unfolding that takes place in this HIC system. For lactalbumin, the unlabeled, light mass peaks in the surface-labeled spectra line up well with the peaks in the no surface control spectra. The positions of the heavy mass, labeled peaks are both close to, but lower than, the fully deuterated mass of lactalbumin (dashed vertical line in Figures 1b and 1c). The existence of two distinct peaks at both buffer conditions and the fact that these peaks occupy very similar positions in both spectra suggest that lactalbumin undergoes a two-state, cooperative unfolding process when adsorbed to Phenyl Sepharose 6 FF high-substitution media.
The exact nature of the lactalbumin structural change is not clear from this data, although it is possible that the more highly-labeled species is not completely unfolded on the resin. The heavy mass peak is located 6 Da lower than the fully deuterated mass in the presence of 12 mM calcium chloride and 3 Da lower in 2 mM EDTA. These differences are small; however, they are equal to or greater than the instrumental error. (The standard deviation of the heavy peak location is 2 Da in the calcium chloride buffer and 1 Da in the EDTA case.) This small amount of protection against hydrogen exchange is likely due to residual native-like, tertiary structure in the molecule. This is consistent with our previous detailed observations of α-lactalbumin structure adsorbed on HIC media both by HX–MS of the intact protein and HX–MS incorporating proteolytic digestion (34). Oroszlan and coworkers (9) observed biphasic unfolding kinetics when studying lactalbumin adsorption and unfolding kinetics on HIC resins, indicating that a kinetic intermediate was involved. Further, lactalbumin has a well-characterized molten globule intermediate in its folding pathway; this intermediate is known to have intact secondary structure but highly disrupted tertiary structure, and it is the dominant structural form of lactalbumin at low pH (42). The unfolded state observed in this study might be close to, but somewhat more compact than, the molten globule form, as a low-pH hydrogen exchange control experiment yielded a mass of 14,256 Da (data not shown). Because fully labeled lactalbumin had a mass of 14,257 Da, it would be very difficult to resolve the molten globule from totally unfolded protein by HX–MS under these labeling conditions.
Another possibility is that the remaining protection against hydrogen exchange observed in the heavy species is a result of exclusion of water from the protein-resin interface. Inherent to this theory is the notion that specific residues in both the folded and unfolded conformations interact with ligands on the resin surface, although the residues involved in binding do not necessarily have to be the same in both conformations. Such behavior has been used to suggest preferential binding of a-lactalbumin on an a more hydrophobic reversed-phase chromatography–like surface (24). While this is a possibility here, HIC surfaces are much less hydrophobic and can be much less effective at excluding water. Additional experiments would have to be performed to confirm this hypothesis.
Conclusions
In this study, amide HX labeling was employed to study conformational changes experienced by hen egg white lysozyme and bovine α-lactalbumin while adsorbed to a hydrophobic interaction chromatography resin. Lysozyme tertiary structure appears to be mainly unchanged in the adsorbed phase, whereas a second, heavier peak appeared in surface-labeled lactalbumin spectra. The fraction of unfolded lactalbumin, as detected by HX and MS, increased when free calcium was removed from the adsorption buffers. The presence of two distinct peaks in the α-lactalbumin mass spectra indicates that the protein adopts one of only two degrees of solvent exposure when adsorbed to Phenyl Sepharose 6 FF high substitution media. The position of the heavy lactalbumin peak is slightly less than the molecular mass of fully labeled lactalbumin, although the difference is similar to instrumental error. The fact that this difference was observed in both the 12-mM calcium and calcium-free cases suggests there can be a small amount of native-like structure retained. This would be consistent with our previous observations of α-lactalbumin adsorption on HIC matrices (34).
Lactalbumin and lysozyme share a high degree of structural homology (41). However, the data presented here show that lactalbumin was highly destabilized on Phenyl Sepharose 6 FF high substitution media while lysozyme was not. This example illustrates the fact that resin selection for chromatographic purification proteins remains a highly empirical process and that the effects of adsorption on stability on a particular resin cannot yet be predicted a priori based upon simple views of structural similarity or sequence. Amide hydrogen-deuterium exchange, as employed in this work, could be a powerful tool for fast resin screening during purification process development, especially when there is a need for detecting and analyzing conformational instability. Moreover, the methods presented here should be applicable to other modes of chromatography such as ion-exchange and affinity chromatography.
Jace L. Fogle and Erik J. Fernandez, Department of Chemical Engineering, University of Virginia Charlottesville, Virginia
Please direct correspondence to Erik Fernandez at erik@virginia.edu
References
(1) W.R. Melander, H.-J. Lin, J. Jacobson, and C. Horvath, J. Phys. Chem. 88, 4527–4536 (1984).
(2) M.M. Hossain and D.D. Do, Chem. Eng. J. 49, B29–B39 (1992).
(3) S.A. Cohen, K. Benedek, S. Dong, Y. Tapuchi, and B.L. Karger, Anal. Chem. 56, 217–221 (1984).
(4) L. Boulkanz, N. Balcar, and M.-H. Baron, Appl. Spect. 49, 1737–1746 (1995).
(5) J.L. McNay and E.J. Fernandez, J. Chromatogr. 849, 135–148 (1999).
(6) J.L. McNay and E.J. Fernandez, Biotech. Bioeng. 76, 224–232 (2001).
(7) S.U. Sane, S.M. Cramer, and T.M. Przybycien, J. Chromatogr. 849, 149–159 (1999).
(8) S.-L. Wu, A. Figueroa, and B.L. Karger, J. Chromatogr. 371, 3–27 (1986).
(9) P. Oroszlan, R. Blanco, X.-M. Lu, D. Yarmush, and B.L. Karger, J. Chromatogr. 500, 481–502 (1990).
(10)M.F.M. Engel, C.P.M. van Mierlo, and A.J.W.G. Visser, J. Biol. Chem. 277, 10922–10930 (2002).
(11) A.A. Shukla, P.J. Hinckley, P. Gupta, Y. Yigzaw, and B. Hubbard, BioProcess Int. 3, 36–45 (2005).
(12) J.D. Lewis and S.L. Nail, Process Biochem. 32, 279–283 (1997).
(13) A. Hvidt and S.O. Nielson, Adv. Protein Chem. 21, 287–386 (1966).
(14) S.W. Englander and N.R. Kallenbach, Quart. Rev. Biophys. 16, 521–655 (1984).
(15) A. Berger, A. Loewenstein, and S. Meiboom, J. Amer. Chem. Soc. 81, 61–67 (1959).
(16) J.J. Englander, D.B. Calhoun, and S.W. Englander, Anal. Biochem. 92, 517–524 (1979).
(17) R.B. Gregory, L. Crabo, A.J. Percy, and A. Rosenberg, Biochem.22, 910–917 (1983).
(18) Y. Bai, J.S. Milne, L. Myne, and S.W. Englander, PROTEINS: Structure, Function, and Genetics 17, 75–86 (1993).
(19) G.P. Connelly, Y. Bai, M.-F. Jeng, and S.W. Englander, PROTEINS: Structure, Function, and Genetics17, 87–92 (1993).
(20) A.K. Covington, R.A. Robinson, and R.G. Bates, J. Phys. Chem. 70, 3820–3824 (1966).
(21) P.K. Glasoe and F.A. Long, J. Phys. Chem. 64, 188–190 (1960).
(22) K. Wuthrich, NMR of Proteins and Nucleic Acids, (Wiley, New York, 1986).
(23) Y. Paterson, S.W. Englander, and H. Roder, Science 249, 755–760 (1990).
(24) M.F.M. Engel, A.J.W.G. Visser, and C.P.M. van Mierlo, Proc. Nat. Acad. Sci. USA 101, 11316–11321 (2004).
(25) Z. Zhang and D.L. Smith, Protein Sci. 2, 522–531 (1993).
(26) L. Wang, H. Pan, and D.L. Smith, Molecular and Cellular Proteomics 1, 132–138 (2002).
(27) L. Wang and D.L. Smith, Anal. Biochem. 314, 46–53 (2003).
(28) L. Wang, L.C. Lane, and D.L. Smith, Protein Sci. 10, 1234–1243 (2001).
(29) Y. Hamuro, S.J. Coales, M.R. Southern, J.F. Nemeth-Cawley, D.D. Stranz, and P.R. Griffin, J. Biomolec. Tech. 14, 171–182 (2003).
(30) S.A. Tobler, N.E. Sherman, and E.J. Fernandez, Biotech. Bioeng. 71, 194–207 (2001).
(31) S.A. Tobler and E.J. Fernandez, Protein Sci. 11, 1340–1352 (2002).
(32) S.A. Tobler, B.H. Holmes, M.E. Cromwell, and E.J. Fernandez, J. Pharm. Sci. 93, 1605–1617 (2004).
(33) S.S. Wang, S.A. Tobler, T.A. Good, and E.J. Fernandez, Biochem. 42, 9507–9514 (2003).
(34) T.T. Jones and E.J. Fernandez, J. Colloid and Interface Sci. 259, 27–35 (2003).
(35) T.T. Jones and E.J. Fernandez, Biotech. Bioeng. 87, 388–399 (2004).
(36) A. Miranker, C.V. Robinson, S.E. Radford, R.T. Aplin, and C.M. Dobson, Science 262, 896–900 (1993).
(37) J.G. Mandell, A.M. Falick, and E.A. Komives, Anal. Chem. 70, 3987–3995 (1998).
(38) S. Ghaemmaghami, M.C. Fitzgerald, and T.G. Oas, Proc. Nat. Acad. Sci. USA 97, 8296–8301 (2000).
(39) M.A. Tito, K. Tars, K. Valegard, J. Hajdu, C.V. Robinson, J. Amer. Chem. Soc. 122, 3550–3551 (2000).
(40) A.A. Rostom, P. Fucini, D.R. Benjamin, R. Juenemann, K.H. Nierhaus, F.U. Hartl, C.M. Dobson, C.V. Robinson, Proc. Nat. Acad. Sci. USA97, 5185–5190 (2000).
(41) Y. Hiraoka, T. Segawa, K. Kuwajima, S. Sugai, N. Murai, Biochem. Biophys. Res. Commun. 95, 1098–1104 (1980).
(42) K. Kuwajima, Y. Ogawa, S. Sugai, J. Biochem. 89, 759–770 (1981).
(43) M. Ikeguchi, K. Kuwajima, S. Sugai, J. Biochem. 99, 1191–1201 (1986).

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











