Analysis of Lipid Nanoparticles
Over the last several years, our definition of biotherapeutics has evolved from solely protein-based therapeutics, such as monoclonal antibodies (mAbs), to include such product classes as cell and gene therapies. Cell and gene therapies are unique products that require innovative and new approaches to formulation, specifically related to drug delivery. One such delivery modality that has gained significant traction is lipid nanoparticles (LNPs), which, as their name suggests, are nanoparticles comprised of lipids. The first LNP product approved by the United States Food and Drug Administration, was the liposome-encapsulated Doxil, whereas, in 2018, the United States Food and Drug Administration approved the small interfering RNA (siRNA) product Onpattro, using lipid nanoparticles as part of its formulation. Since then, LNPs have gained more international attention in their role in drug delivery because of the mRNA-based COVID-19 vaccines. LNPs in the biopharmaceutical space are still in their infancy related to our understanding of them and how to characterize them, presenting both challenges and opportunities. In this column, we discuss some of those challenges and opportunities and look at the current and future landscape of the most common analytical tools used to analyze and characterize LNPs.
As our understanding of biotherapeutics expands to include new products such as cell and gene therapies, we are presented with new challenges and opportunities in the characterization of these products. These new product classes also present challenges and opportunities in formulation, especially related to drug delivery. One of the emerging strategies for drug delivery is using lipid nanoparticles as the transport system for drugs.
The precursor to the current lipid nanoparticle (LNP) delivery systems was the liposome (Figure 1a). Liposomes are typically nanosized, closed lipid bilayer vesicles that can form spontaneously in water, which has led most to consider them the first generation of LNPs (2). They were recognized almost immediately after their discovery as a potential drug delivery system for both hydrophobic (not water soluble) and hydrophilic (water soluble) drugs. In particular, drugs that are not water soluble, of which approximately 40% of small-molecule cancer drugs are, would be discarded because they lack a delivery method, but liposomes presented an opportunity to deliver these molecules without worrying about solubility issues (2). Liposomes, which range in size from 20 to ~1000 nm, are most commonly made up of phosphatidylcholines, phosphatidylethanolamines, phosphatidylserine, phosphatidylglycerol, and other stabilizers (for example, cholesterol). Hydrophilic drugs are typically enclosed in the aqueous interior of the lysosome, whereas hydrophobic drugs can be found in the phospholipid bilayer itself. The first FDA-approved liposomal drug was Doxil, a treatment for ovarian cancer, in 1995 (1,2).
Figure 1: Schematics of different classes of lipid-based nanoparticles: (a) liposomes, (b) SLNs, (c) NLCs, and (d) hybrid lipid-polymeric nanoparticles (Open Access [1]).
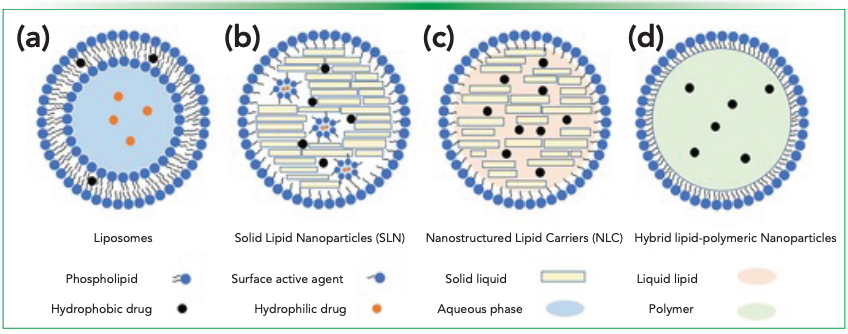
Over time, lipid nanoparticles evolved from liposomes to other formulations, such as solid lipid nanoparticles (SLN), nonstructured lipid carriers (NLC), and hybrid lipid-polymeric nanoparticles. SLNs (Figure 1b) are made up of fully crystallized lipid components with drugs encapsulated in a highly ordered crystalline structure with stabilizing molecules. There are several advantages to SLNs, such as improved stability, controlled release, drug protection and others; however, there are also several disadvantages, such as poor longterm drug retention and low drug loading capacity. NLCs (Figure 1c) build on SLNs by substituting liquid lipids for the solid lipids, which resulted in a larger drug distribution space; thus, they lead to better drug retention and drug loading capacity. Further improvements were made to LNPs with hybrid LNPs (Figure 1d). Hybrid LNPs, for example, may contain a therapeutic-containing polymeric core encapsulated by an inner lipid layer and a PEGylated lipid outer layer (1). Other examples of LNPs include cationic LNPs (complexes with nucleic acids), nonlamellar LNPs (hexosomes), and ethosomes (consisting of ethanol) (2). Onpattro was the first U.S. FDA approved double-stranded small interfering RNA (siRNA) drug that used LNPs for its delivery (1).
Synthesis of Lipid Nanoparticles
With this general understanding of what LNPs are, it is also important to consider how they are synthesized or made. Understanding how LNPs are made will help us better understand their characterization. Synthesis of LNPs include a number of techniques to control their size, numbers of concentric bilayers (lamellarity), and their ability to encapsulate various drugs (2).
The oldest, and perhaps easiest, method of LNP preparation is the film hydration method. Using this method, lipids are dissolved in an organic solvent and are dried down, resulting in a thin film that is then hydrated, creating a liposomal dispersion. Gentle hydration leads to giant unilamellar vesicles, whereas intense agitation generates multilayer vesicles (2). Another process that can be used for LNP preparation is reverse-phase evaporation. Reverse-phase evaporation involves the formation of a water-in-oil emulsion between an aqueous and organic phase containing lipids (2). More recently, microfluidic hydrodynamic focusing has been used to formulate LNPs. In this approach, a stream of lipid in alcohol is forced to flow through a central channel (microchannel) where it is intersected and sheathed by coaxial streams of an aqueous phase, which causes the lipid to precipitate and form liposomes (1,2).
There are several other methods that can be used to formulate LNPs, in particular for SLNs and NLCs: solvent-based emulsification, nonsolvent emulsification, bulk nanoprecipitation, and coacervation. In solvent-based emulsification solid lipids are dissolved in water-immiscible organic solvent, which forms oil-in-water emulsions when they are dispersed in an aqueous solution. The evaporation of the organic solvent produces LNPs. Nonsolvent emulsifications use melted lipids to form the oil-in-water emulsions. In bulk nanoprecipitation, a water-miscible solvent containing lipids mixes with an aqueous phase, and, through rapid desolvation, leads to rapid precipitation, creating LNPs. Coacervation uses a micellular solution of fatty acid alkaline salts, which is precipitated as pH is lowered, thus creating LNPs. This approach is solvent-free and does not require sophisticated equipment or harsh solvents. All these strategies, and others not mentioned here, are useful for small-scale production of LNPs. As for large-scale production of LNPs, the two strategies used are nonsolvent and solvent based emulsification (1).
Characterization of Lipid Nanoparticles
Characterization of LNPs is often accomplished by a comprehensive characterization of the entire make-up (individual components and formulated LNP) of the LNP. That is, there is the analytical characterization of the lipids themselves and the active pharmaceutical ingredient (API). Then, there is the characterization of the formulated individual nanoparticle (lipids + API + excipients) and the nanoparticle emulsion (solution of all LNPs). Lastly, there is the characterization of the release of the API from the LNP. Briefly, lipid characterization includes the identification and quantification of the lipids; API characterization includes total API and the amount of encapsulated or unencapsulated API; nanoparticle characterization includes size, charge, and structure; nanoparticle mixture characterization is focused on physical and chemical stability; and characterization of release is based on the release kinetics of the API (3). The below discussions center on the characterization of the individual nanoparticles and nanoparticle mixtures.
Individual Components: Lipids and Active Pharmaceutical Ingredients
A part of the characterization of LNPs is the individual characterization of the lipids themselves and the active pharmaceutical ingredients. In this column, we focus solely on the characterization of the lipids themselves. We will leave a discussion of API characterization to others.
There are numerous analytical tools that can be used to characterize lipids. Nuclear magnetic resonance (NMR) can be used to characterize lipids in LNPs. Specifically, NMR can measure the chemical shifts of the observed nucleus with unique electron density in a lipid, thus providing information on the molecular structure of alkyl tails and lipid head groups as an example. Vibrational spectroscopy has been used to identify lipid components, as well (3).
Mass spectrometry (MS) can be used to elucidate the molecular mass of lipids by using soft ionization techniques with electrospray ionization. MS coupled to liquid chromatography (LC–MS) provides for lipid separation and identification (3–6). Reversed-phase liquid chromatography (RPLC), and RPLC–MS, has been used for decades to quantitate lipids (3,7,8). In addition, the development of mRNA-based Covid-19 vaccines encapsulated in LNPs for delivery has led to the rapid development of novel LC–MS approaches to characterize the lipids as seen by publication of several recent application notes (9,10). In addition to a mass spectrometer for detection, other detectors such as charged aerosol detectors (CAD), refractive index (RI), and ultraviolet (UV) diode array can be used (3).
Formulated Lipid Nanoparticles (Lipids with API)
Characterization of formulated LNPs uses a variety of different analytical tools. Unlike characterization of other biomolecules discussed in previous columns, physical evaluation is a major component of LNP characterization. As LNPs are small nanoparticles, one important characteristic to consider is size and shape. To make the sizes and shapes of LNPs visible, a commonly used technique is electron microscopy, which allows for visualization of the shape, inner structure and surface of a LNP. Scanning electron microscopy (SEM) provides input into shape and size distribution of dry microparticles. SEM also allows for characterization of the outer and inner structure, thus providing information about the components of the LNP. Cryogenic transmission electron microscopy (Cryo–TEM) can be used to characterize liquid dispersions of LNPs, which provides information on morphology and inner details, where the drug products are in a state very close to their native state. In addition, the scale bar provided in cryo–TEM experiments provides information on size, thus providing an additional tool to differentiate different morphological LNPs. Therefore, microscopy of LNPs provides information about the size, shape and distribution of particles. However, when using microscopy, like with many analytical tools, sample preparation is crucial to ensure appropriate characteristics are observed (11).
In addition to size and shape, understanding the internal make-up (structural organization) of LNPs is important. Lipids are known to be sensitive to temperature, water concentration, and the presence of different compounds, thus resulting in aggregation. To characterize this aspect of LNPs, X-ray diffraction, a historical technique used to characterize lipid dispersion, is currently the most common approach. In being able to characterize the structural organization of LNPs, X-ray diffraction can also provide information on how the drug molecules encapsulated in the LNPs affect the overall structure of LNP nanosystems. The understanding of the structural organization, including the effect of the drug molecule, is important to ensure the stability of the LNP nanostructure that allows for appropriate delivery of the molecule to the target as well as ensuring aggregation, which can cause an immunogenic effect, will not be an issue. Cryo–EM and X-ray diffraction results are complimentary, providing confirmatory information, on morphology and structural information (11).
Along with cryo–EM, other complimentary techniques are often used to characterize the size of nanoparticles, which are usually in the 10 to 200 nm size range. Of note, analytical ultracentrifugation and flow field fractionation are often not used, because of too minuscule particle sizes stemming from particle charges. Thus, more common techniques used include sedimentation field flow fractionation (SdFFF), size exclusion chromatography (SEC), dynamic light scattering/photon correlation spectroscopy (DLS-PCS), and cryo–EM (which has been discussed previously). SdFFF characterizes the size distribution of LNPs based on gravitational or centrifugal force (11). SEC can be used to purify drug-loaded nanoparticles (preparative SEC), and analytical SEC can be used to determine particle size distributions. An SEC column, depending on pore size, allows for larger molecules to elute faster than smaller counterparts and, when paired with appropriate standards, can give a relative size (3). DLS-PCS can measure the diameter (dimensions) of LNPs, where intensity of scattered light is determined as a function of time, to distinguish nanoparticles from bulk materials. The dimensions, as well as surface charge, are important aspects of LNP characterization because they are both implicated in cellular uptake, toxicity and dissolution. Of note, scattered light through polydisperse samples is difficult to characterize, as the intensity of light through bigger particles is more intense than smaller ones; thus, DLS-PCS should not be used to characterize polydisperse nanosystems. In the case of polydisperse nanosystems, cryo-EM is more appropriate (see above) (11).
An understanding of zeta potential and surface charge are also important aspects to the characterization of LNPs, as they can affect such things as solubility (aggregation). Zeta potential provides information on colloid-electrolyte interactions, and is based on the fact that oppositely charged particles are attracted to each other while similarly charged particles are repelled (12). Zeta potential is measured using electrophoretic techniques. Measuring zeta potential can be very challenging, and is affected by dilution of the suspensions; thus, for every new formulation, characterization should be done to determine the appropriate conditions to measure zeta potential. Currently, there is no direct way to measure surface charge; thus, surface charge is measured by making an electric field around the particles and measuring voltage (11). Generally, zeta potential and surface charge are measured separately, which can add complexity to LNP characterization. That said, there are methods being developed to measure both together, such as the example by Rasmussen and coauthors that uses a one-step measurement with a salt gradient and capillary to measure zeta potential and surface charge (13).
Conclusion
This column introduced, briefly, different types of lipid nanoparticles (LNPs), how they are synthesized, and how they are characterized. Characterization of pharmaceuticals, such as mRNA encapsulated in LNPs, includes 1) characterization of the lipids; 2) characterization of the active pharmaceutical ingredient; 3) characterization of the formulated (lipids + API) LNPs; 4) characterization of the LNP populations; and 5) characterization of API release from the LNPs. There is diversity in the techniques used to accomplish these characterization steps. Such things as cryo–EM are used to characterize the formulated LNP product. Other, more traditional biopharmaceutical analytical tools like LC–MS are used to characterize the lipids and the API, separately. The field of LNP products and analysis is still in its early stages; however, the mRNA Covid-19 vaccines have catalyzed efforts to develop new tools and methodologies to characterize LNP products. There is significant opportunity for research and development in this space. As such, this column serves as a brief overview of the current stage, but we envision rapid development in the characterization of LNPs, providing opportunities for future columns to take a deeper dive into individual analytical technologies.
References
(1) Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R. J.; Zhao, C.-X., Lipid Nanoparticles for Drug Delivery. Advanced NanoBiomed Research 2022, 2, 1–17. DOI: https://doi.org/10.1002/anbr.202100109
(2) Tenchov, R.; Bird, R.; Curtze, A. E.; Zhou, Q., Lipid Nanoparticles--From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. DOI: https://doi.org/10.1021/acsnano.1c04996
(3) Fan, Y.; Marioli, M.; Zhang, K., Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. DOI: https://doi.org/10.1016/j.jpba.2020.113642
(4) Harkewicz, R.; Dennis, E. A., Applications of Mass Spectrometry to Lipids and Membranes. Annu. Rev. Biochem. 2011, 80, 301–325. DOI: https://doi.org/10.1146/annurev-biochem-060409-092612
(5) Knittelfelder, O. L.; Weberhofer, B. P.; Eichmann, T. O.; Kohlwein, S. D.; Rechberger, G. N., A versatile ultra-high performance LC-MS method for lipid profiling. J. Chromatogr. B 2014, 951-952, 119-28. DOI: https://doi.org/10.1016/j.jchromb.2014.01.011
(6) Scherer, M.; Bottcher, A.; Liebisch, G., Lipid profiling of lipoproteins by electrospray ionization tandem mass spectrometry. Biochim. Biophys. Acta. 2011, 1811 (11), 918–924. DOI: https://doi.org/10.1016/j.bbalip.2011.06.016
(7) Cajka, T.; Fiehn, O., Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trends Analyt. Chem. 2014, 61, 192–206. DOI: https://doi.org/10.1016/j.trac.2014.04.017
(8) Christie, W. W., Rapid separation and quantification of lipid classes by high performance liquid chromatography and mass (light-scattering) detection. J. Lipid Res. 1985, 26 (4), 507–12. DOI: https://doi.org/10.1016/S0022-2275(20)34367-4
(9) Isaac, G.; Ranbaduge, N.; Alden, B. A.; Quinn, C.; Chen, W.; Plumb, R. S. Rapid Analysis of Lipid Nanoparticle Components Using BioAccord LC-MS System. Waters Corporation, 2021. https://www.waters.com/content/dam/waters/en/app-notes/2021/720007296/720007296-en.pdf (accessed 2023-05-10)
(10) Schneider, S., Analysis of Lipid Nanoparticle Composition. Agilent Technologies, 2021. https://www.agilent.com/cs/library/applications/an-elsd-1260-infinity%20II-prime-bio-lc-1290-infinity%20II-lcsd-5994-4709en-agilent.pdf (accessed 2023-05-10)
(11) Hallan, S. S.; Sguizzato, M.; Esposito, E.; Cortesi, R., Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics 2021, 13 (4). DOI: https://doi.org/10.3390/pharmaceutics13040549
(12) Rajpoot, K.; Tekade, R. K., Microemulsion as drug and gene delivery vehicle: an inside story. In Drug Delivery Systems, Advances in Pharmaceutical Product Development and Research; Academic Press, 2019; pp 455–520.
(13) Rasmussen, M. K.; Pedersen, J. N.; Marie, R., Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11 (1), 2337. DOI: https://doi.org/10.1038/s41467-020-15889-3
About the Author
Jared Auclair is an Associate Dean of Professional Programs and Graduate Affairs at the College of Science at Northeastern University, in Boston, Massachusetts. He is also the Director of Biotechnology and Informatics, as well as the Director of the Biopharmaceutical Analysis Training Laboratory.

About the Column Editor
Anurag S. Rathore is a professor in the Department of Chemical Engineering at the Indian Institute of Technology in Delhi, India.


Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)