Analysis of Dioxins in Foods and Feeds Using Gas Chromatography Tandem Mass Spectrometry
The Column
In the early days of dioxin analysis, applied methods were laboratory- and time-consuming. Only GC–HRMS, which is complicated, was used. Nowadays, GC–MS/MS is suitable for control proposes. Using GC–MS/MS means that solvent consumption for sample preparation can be reduced by a factor of 10 and the purity of the obtained fraction can be enhanced, indicating that GC–MS/MS is appropriate for dioxin analysis.
Photo Credit: Piman Khrutmuang/stock.adobe.com

In the early days of dioxin analysis, applied methods were laboratory- and time-consuming. Only gas chromatography–high resolution mass spectrometry (GC–HRMS), which is complicated, was used. Nowadays, GC–MS/MS is suitable for control proposes. Using GC–MS/MS means that solvent consumption for sample preparation can be reduced by a factor of 10 and the purity of the obtained fraction can be enhanced, indicating that GC–MS/MS is appropriate for dioxin analysis.
Persistent organic pollutants (POPs) are semivolatile and can be detected worldwide, even in remote regions. They bioaccumulate with potential negative influence on the environment and human health. To a varying degree, these organic compounds resist photolytic, biological, and chemical degradation.
POPs in foods and feeds can be analyzed with several methods. Dioxins are particularly toxic, even for POPs, so quantitative analysis is required down to low concentrations. Until recently, the analysis of dioxins was performed using gas chromatography–high resolution mass spectrometry (GC–HRMS), which provides highly accurate quantitation. However, triple quadrupole GC–MS/MS is less expensive and easier to handle than GC–HRMS, so its use is increasingly being investigated.
In recent years, the quantitative accuracy of GC–MS/MS has improved significantly. Accordingly, the use of this analysis method has been officially recognized in the EU (EU589/2014, 644/2017). However, in order to change from GC–HRMS to GC–MS/MS, it is necessary to compare their respective quantitative abilities.
In this article, dioxins (polychlorinated dibenzo-p-dioxin [PCDD] and polychlorinated dibenzofuran [PCDF] only) were analyzed in 44 types and 200 samples of foods and feeds using GC–MS/MS. Additionally, GC–MS/MS analysis results were compared with those from GC–HRMS in order to evaluate the quantitative capabilities of both techniques.
Experiment
For the various food samples, pretreatment was performed using an automatic pretreatment unit (extraction: Speed Extractor [Buchi]; purification: GO-xHT [Miura Co., Ltd.]).
System Configuration: Autosampler: AOC-20i/S; GC–MS/MS system: GCMS-TQ-8050 NX; software: GCMSsolution Ver 4.45 SP1, LabSolutions Insight Ver. 3.2 SP1, GC–MS/MS method package for dioxins in food (all Shimadzu).
Analytical Conditions (Autosampler): No. of rinses with solvent (pre-run): 3; no. of rinses with solvent (post-run): 3; no. of rinses with sample: 0; washing volume: 6 µL; injection volume: 2 µL; viscosity comp. time: 0.2 s.
A 10-µL measure of nonane was used as final solvent for the samples. For the standard, a mixture of DF-ST and DF-LCS, commercially available standard mixtures, from Wellington Laboratories was used. In terms of the analytical conditions for GC–MS/MS, the conditions registered in the method package were used.
Analytical Conditions (GC): Insert liner: Topaz single gooseneck liner with wool; column: 60 m × 0.25 mm, 0.25âµm SHâRxi-5Sil MS (Shimadzu); injection mode: splitless; sampling time: 1.00 min; injection temperature: 280 °C; column oven temperature: 150 °C (1 min) > (20 °C/min) > 220 °C > (2 °C/min) > 260 °C (3 min) > (5 °C/min) > 320 °C (3.5 min); HP injection: 450 kPa (1.5 min); flow control mode: linear velocity (45.6 cm/s); purge flow: 20 mL/min; carrier gas: helium.
Analytical Conditions (MS): Ion source temperature: 230 °C; interface temperature: 300 °C; detector voltage: 1.8 kV (absolute).
Results
Analysis Results for the Standards: In the analysis of dioxins in foods, maximum levels (MLs) are prescribed for each sample. With the food and feed samples in this investigation, MLs for pig fat and pig meat were the lowest at 1 pg/g of fat. In addition, the limit of quantitation (LOQ) required for each compound in the analysis depends on the sample’s ML, the pretreatment method, and the toxic equivalence factor (TEF) of each compound. The compounds 2,3,7,8-Tetrachlorodibenzo-p-dioxin and 1,2,3,7,8-Pentachlorodibenzo-p-dioxin have the highest TEF (TEF = 1), requiring lower LOQs than other compounds. In this investigation, the LOQ for both dioxins in pig fat and pig meat was 0.060 pg/µL at the concentration in the final vial.
Signal-to-Noise (S/N) Ratio (“Method 1”): The concentration of an analyte in the extract of a sample that produces an instrumental response at two different ions to be monitored with a S/N ratio of 3:1 for the less intensive raw data signal.
Lowest Concentration Point on the Calibration Curve (“Method 2”): The lowest concentration point on a calibration curve that gives an acceptable (≤ 30%) and consistent (measured at least at the start and at the end of an analytical series of samples) deviation from the average relative response factor calculated for all points on the calibration curve in each series of samples. In this article, for the purposes of confirmation, an evaluation was performed using both criteria.
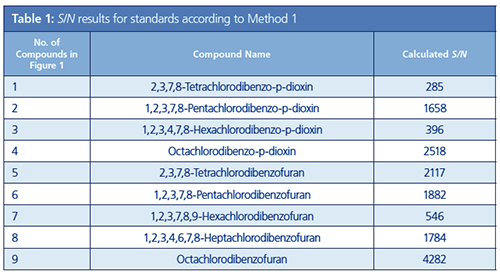
As noted above, for 2,3,7,8-Tetrachlorodibenzo-p-dioxin it is necessary to set the LOQ to 0.060 pg/µL or less. Accordingly, the prepared standard solution (STD) was prepared so that the concentration of each compound was 0.050 pg/µL (for Octachlorodibenzo-p-dioxin and Octachlorodibenzofuran 0.100 pg/µL). From the results of the analysis, it was evident that the criteria for Method 1 were satisfied for all compounds. S/N ratios for each compound are shown in Figure 1 and Table 1.
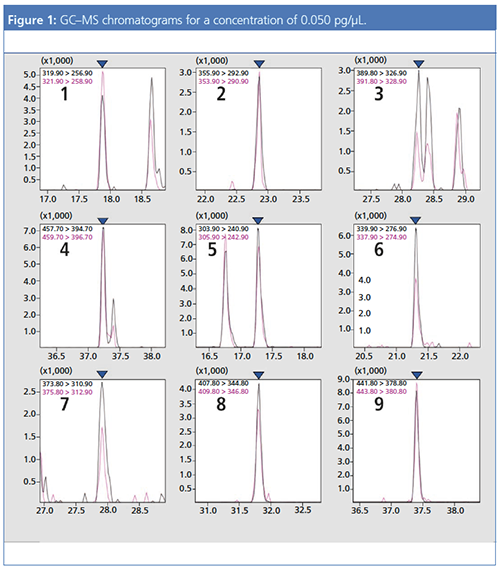
With Method 2, a calibration curve was created with all six levels used, including 0.025 pg/µL, 0.050 pg/µL, 0.100 pg/µL, 0.250 pg/µL, 0.500 pg/µL, and 1.000 pg/µL. The concentrations for each compound at each calibration curve point (level) are shown in Table 2. For each compound, when the level 1 RRF and average RRF were compared, it was found that all compounds satisfied the criteria for Method 2. From the above-mentioned results, it was evident that at the LOQ,
the criteria were satisfied for all compounds.
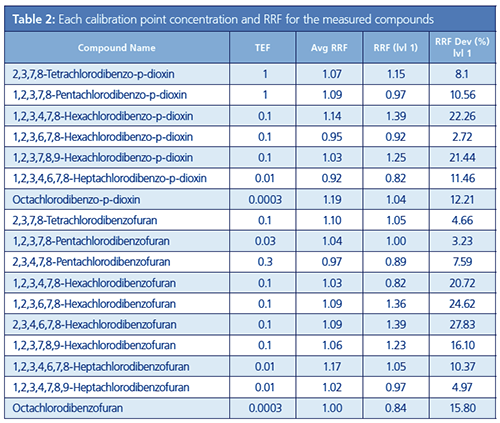
Analysis Results for the Test Samples: As previously noted, the level of toxicity differs for each dioxin compound. The TEF, calculated for each compound by taking the toxicity of 2,3,7,8-Tetrachlorodibenzo-p-dioxin as 1, is used as an index of strength. The TEF values for each compound are shown in Table 2. The ML for the dioxins in foods and feeds are prescribed by their toxic equivalents (TEQ). The TEQ is calculated by multiplying the concentration of each compound by the TEF and then calculating the total TEQ for all compounds.
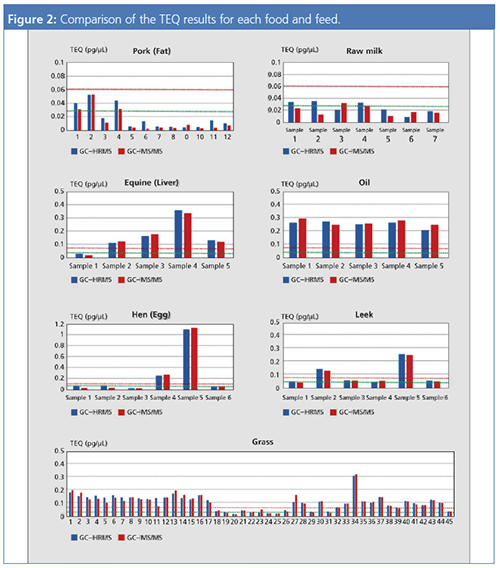
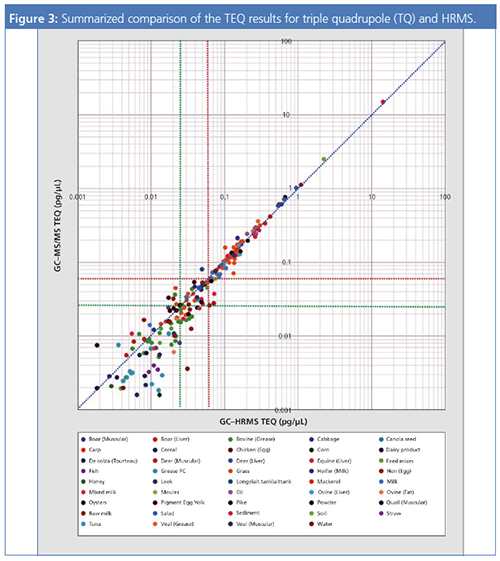
Figure 2 and Figure 3 show a comparison of the TEQ values for GC–MS/MS and GC–HRMS for food samples. A TEQ of 0.060 pg/µL (red line) and a TEQ of 0.025 pg/µL (green line) are marked as indicators for the samples.
Conclusion
In this article, dioxins were analyzed in 44 types and at least 200 samples of foods and feeds using GC–MS/MS. In addition, the GC–MS/MS analysis results were compared with the analysis results from GC–HRMS in order to assess the quantitative capabilities of both methods. Before analyzing, a STD was measured using GC–MS/MS, and it was confirmed that the criteria were satisfied at the LOQ.
From the above-mentioned results, it is evident that GC–MS/MS analysis provides a quantitative capability equivalent to that of GC–HRMS for samples at the concentration levels required for analysis. However, at concentrations below the required level, differences in quantitative capability could arise. For this reason, it is necessary to confirm quantitative capability at the LOQ, and evaluate whether there has been a decrease in sensitivity.
Waldemar Weber started his study as a chemist in 2005 at the University of Münster, Germany. After graduation as a chemist, he did his Ph.D. at the University of Münster in 2011, and finished as a postdoc in 2013 at the Research Center “MEET” in Münster, Germany. From 2012 to 2013 he was employed as an Application Specialist at JAS in Moers, Germany. In November 2018 he joined Shimadzu Europa as Product Manager for
GC–MS.
Masato Takakura works at Shimadzu Europa GmbH.
Thomas Lehardy works at LABERCA in Nantes, France.
Philippe Marchand works at LABERCA in Nantes, France.
E-mail:shimadzu@shimadzu.euWebsite:www.shimadzu.eu

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.








