The Advent and Potential Impact of Ionic Liquid Stationary Phases in GC and GCxGC
LCGC Europe
This article discusses the unique properties of ILs and their potential impact as GC stationary phases.
Choices of available stationary phases for gas chromatography (GC) have been fairly constant for many years. The same basic types of columns that do analogous separations can be obtained from any of a large number of sources, worldwide. Recent research has indicated that unique new substances have been developed that will play an important role in GC column technology. These substances are ionic liquids (ILs). ILs are solvents in which the constituents consist entirely of ions. By definition, they are pure salts that have melting points below 100 °C. However, when used as GC stationary phases, melting points in the range of ~ –40 °C to 50 °C are preferable. ILs have a number of properties that make them exceptional stationary phases. For example, their viscosity can be varied over a broad range, they can have high thermal stabilities, they can be coated on fused-silica capillaries with high efficiencies, they have unique solvent properties and they can be immobilized and crosslinked.1–7 Indeed, it was noted early on that IL stationary phases had a dual nature in that they separated nonpolar analytes as if they were nonpolar stationary phases and simultaneously separated polar analytes as if they were polar stationary phases.8

KEY POINTS
Another very important aspect of IL stationary phases is that their physico-chemical properties are almost infinitely tunable. Tunability is a characteristic that is unavailable to all other classes of GC stationary phases. With relatively simple synthetic modifications or changes to an IL's cation, anion, the substituents thereon and their linkage chains, one can alter and control whatever solvent and selectivity characteristics that are desired.9–11
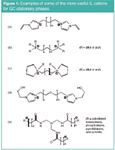
Figure 1
Types of ILs for GC
Figure 1 shows the structures of typical "tunable," high-stability cations that have been shown to be particularly useful as GC stationary phase components. Figure 2 shows the structure of two of the more common anions used in IL–GC formulations. The bis(trifluoromethane)sulphonamide anion (NTf2–) tends to produce ILs with lower melting points and somewhat lower polarities than the triflate anion (TfO– ). Also, it provides excellent peak shapes for nonhydrogen bonding or weakly hydrogen bonding analytes, but tends to produce tailing peaks for alcohols, carboxylic acids and amines. The tailing peaks of these strong hydrogen bonding analytes can be minimized or eliminated by masking the effect of the NTf2– anion by using a cation containing an amide moiety [see trigonal cation in Figure 1(e)] or by using the triflate anion. Examples of these behaviours will be shown throughout this monograph. The cations (Figure 1) can be further selected and varied to emphasize or deemphasize any known solvation interactions including: n/π, dipolar, H-bond acidity, H-bond basicity and dispersion interactions. Clearly, the hydrocarbon linkage chains (connecting the charged moieties) produce less polar stationary phases than polyethylene glycol types. Shorter linkage chains result in more polar stationary phases than analogous longer chains. Imidazolium cations have a delocalized positive charge in contrast to phosphonium and pyrrolidinium cations [Figures 1(b) and 1(c)]. The NTf22– anion has a more delocalized charge and is more hydrophobic than the triflate anion.
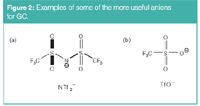
Figure 2
While it is difficult to predict the future, it is clear that IL stationary phases will have a direct impact on specific areas of GC. Four representative areas are listed in Table I. Examples of IL-based separations involving each of these "impact areas" will be presented and discussed.

Table 1: Areas where ionic liquid stationary phases will impact GC.
Polar IL Stationary Phases with Low Bleed and High Thermal Stability
Polyethylene glycol (PEG) wax-type coatings are popular and ubiquitous polar stationary phases in GC. The "bleed temperature" can vary somewhat with the molecular weight of the polymers used, and possibly with the pretreatment procedures used on the fused-silica capillary. Typically, the upper temperature limits for these columns are in the 240–280 °C range, depending upon the manufacturer. Figure 3 shows a comparison of the thermal stability–bleed profiles between a typical PEG GC column and a phosphonium IL-based column of approximately the same polarity [see structure in Figure 1(b)]. The "wax" stationary phase starts to show significant bleed at 280 °C and by 350 °C this stationary has been stripped completely from the fused-silica capillary. At the same temperature (350 °C), the phosphonium IL column is just beginning to have detectable bleed. Indeed the lower bleed of many IL columns and smaller, simpler fragmentation at high temperatures results in less interference and lower limits of detection for many GC–mass spectrometry (MS) applications. Figure 4 shows that there are minimal changes in the retention times of a rapeseed oil fatty acid methyl ester (FAME) mixture on the phosphonium IL column after 80 h of conditioning at 300 °C.

Figure 3
Polar IL Stationary Phase with Same Selectivity and Higher Stability
One of the most polar GC stationary phases available today is 1,2,3-tris(2-cyanoethoxy)propane (TCEP) (Figure 5). However, it has a maximum operating temperature of 140 °C and is moisture and oxygen sensitive. However, a divinylimidazolium NTf2– IL has been engineered to give the same selectivity (Figure 6), and it is both oxygen and moisture-stable and has a thermal stability of 240 °C. In addition, the IL column (SBIL-100, Supelco, Bellefonte, Pennsylvania) shows unique selectivities for many other types of compounds. Unique selectivity is shown by the differences in the retention order of PCB congeners on the IL column (Figure 7) versus a commercial cyanopropylpolysiloxane column (SP-2331, Supelco, Bellefonte, Pennsylvania). Also note the faster elution times on the IL column, observed for a different standard mixture versus the TCEP column (Figure 6).

Figure 4
IL Stationary Phases with New Selectivities
IL stationary phases also appear to have excellent selectivity for cis–trans isomers of FAMEs, as shown in Figure 8. Positional geometric FAME isomers typically are resolved using 100 m × 0.25 mm highly polar cyanosilicone stationary phases. Figure 8 shows that a 60 m × 0.25 mm SLB-IL100 column is capable of providing resolution of a number of the monoene, diene and triene fractions of the C18 FAME isomers. Better resolution is seen of the triene compared with that provided by the longer cyanosilicone column.12
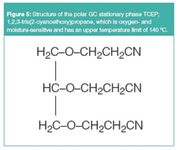
Figure 5
Perhaps the most polar IL stationary phases yet tested are based upon hydroxyimidazolium (PEG) triflate [Figure 1(d)]. In addition to being highly polar and possessing unique selectivities, the triflate anion gives excellent peak shapes for analytes with strong hydrogen bonding groups (especially alcohols, diols, carboxylic acids and amines). This feature is illustrated in Figures 9 and 10. A chromatogram for a mixture of n-alkanes and alcohols is shown in Figure 9. Note how much the hydrocarbons are shifted to lower retention values relative to the alcohols. Also note the excellent peak shapes for both groups of analytes on this relatively short 10 m column. The Grob mix is separated on the same 10 m column as shown in Figure 10. Because of the triflate anion, the acidic and basic analytes (peaks 6, 7, 11 and 12) have good peak shapes. Also note that peak 8 (methyl decanoate) is eluted before peaks 4 and 3 (that is, 1-octanol and 1-nonanal). This is quite unusual and is indicative of the high polarity of this IL stationary phase.

Figure 6
IL Stationary Phases for Comprehensive GC
Given the unique characteristics of IL stationary phases, it is apparent that they will be quite useful for comprehensive multidimensional separations. Recent reports13,14 involving GC×GC indicate that IL columns used in conjunction with conventional columns (in either the first or second dimension) show unique group selectivities. For example, when analysing diesel fuel with GC×GC, the best group separation of saturated hydrocarbons versus monoaromatic versus diaromatic compounds was obtained when using a phosphonium NTf2– IL in the first dimension and a nonpolar HP-5 column in the second dimension.13

Figure 7
In other work, a GC×GC separation (which used a divinylimidazolium triflate IL in the second dimension) was the only combination able to completely isolate analytes containing phosphorus–oxygen moieties from all other compounds.14 This type of group selectivity is particularly important when analysing pesticides, nerve agents and residues thereof. Tunable dual-column GC with a divinylimidazolium NTf2– stationary phase was used in conjunction with headspace sampling to characterize US currency and other complex samples.15

Figure 8
Figure 11 is a comparison of the GC×GC–TOF-MS profiles of a petroleum distillate using two different column sets.16 Figure 11(b) used a phosphonium NTf2– column in the second dimension rather than the conventional PEG–wax column [Figure 11(a)]. As can be seen, the important toxic compound, diphenylmethane, is only resolved when using the IL column [Figure 11(b)]. In addition, it appears that the group separations are more definitive in the column set that uses the IL stationary phase.

Figure 9
Conclusions
Undoubtedly, 2009 will be a "benchmark" year for ILs in GC. They will become widely available to both academic and industrial scientists for the first time. Although ILs will continue to be the focus of cutting-edge research in separation science in the foreseeable future, the current IL materials are about to be thoroughly examined and tested by the everyday users of GC. They will be doing things to and with these columns that were not the focus of academics. Through this coming harsh reality, we will learn considerably more about the uses and limitations of IL stationary phases. These lessons will then lead to another generation of ILs; because after all, they are almost infinitely tunable.

Figure 10

Figure 11
Daniel W. Armstrong is a Robert A. Welch Professor. He is known for developing the theory and practice of novel and effective methods of chemical analysis. He first introduced micelles and cyclodextrins to analytical chemistry and made fundamental advances in the mechanism and use of chiral separations including the first commercial reversed-phase columns. He synthesized, characterized and evaluated the greatest variety of effective ILs for chemical analysis.
D.W. Tharanga Payagala is a senior graduate student in Dr Armstrong's research group at the University of Texas at Arlington. She is working on design, synthesis and evaluation of Ionic Liquids and synthesis of novel chiral and non-chiral GC and HPLC stationary phases.
Leonard M. Sidisky received his BS degree in Biology and an MSc in Food Science from The Pennsylvania State University. He is presently the R&D Manager for Gas Separations at Supelco (division of Sigma Aldrich). His research interests are in the development of gas chromatographic products for wide range of industrial applications, theory and practical application of capillary gas chromatography, and solid phase micro extraction (SPME) product development and applications.
References
1. X. Han and D.W. Armstrong, Acc. Chem. Res., 40, 1079 (2007).
2. J.L. Anderson and D.W. Armstrong, Anal. Chem., 77, 6453 (2005).
3. K. Huang, X. Han, X. Zhang and D.W. Armstrong, Anal. Bioanal. Chem., 389, 2265 (2007).
4. Z.S. Bretibach and D.W. Armstrong, Anal. Bioanal. Chem., 390, 1605 (2008).
5. T. Payagala et al., Anal. Chem., 81, 160 (2009).
6. J.L. Anderson, D.W. Armstrong and G.T. Wei, Anal. Chem., 78, 2893 (2006).
7. R.J. Soukup-Hein, M.M. Warnke and D.W. Armstrong, Annu. Rev. Anal. Chem., 2, 8.1 (2009).
8. D.W. Armstrong, L.F. He and Y.S. Liu, Anal. Chem., 71, 3873 (1999).
9. T. Payagala et al., Chem. Mater., 19, 5848 (2007).
10. J.L. Anderson et al., J. Am. Chem. Soc., 124, 14247 (2002).
11. J.L. Anderson, R. Ding, A. Ellern and D.W. Armstrong, J. Am. Chem. Soc., 127, 593 (2005).
12. C. Ragonese et al., Anal. Chem., in press (2009).
13. J.V. Seeley et al., Anal. Bioanal. Chem., 390, 323 (2008).
14. V.R. Reid, J.A. Crank, D.W. Armstrong and R.E. Synovec, J. Sep. Sci., 31, 3429 (2008).
15. G.R. Lambertus et al., J. Chromatogr. A., 1135, 230 (2006).
16. J.M.D. Dimandja et al., Pittcon 2009 Technical Program, poster 1980-26P, March 11, 2009.
Analysis of Pesticides in Foods Using GC–MS/MS: An Interview with José Fernando Huertas-Pérez
December 16th 2024In this LCGC International interview with José Fernando Huertas-Pérez who is a specialist in chemical contaminants analytics and mitigation at the Nestlé Institute for Food Safety and Analytical Sciences at Nestlé Research in Switzerland, In this interview we discuss his recent research work published in Food Chemistry on the subject of a method for quantifying multi-residue pesticides in food matrices using gas chromatography–tandem mass spectrometry (GC–MS/MS) (1).
The Use of SPME and GC×GC in Food Analysis: An Interview with Giorgia Purcaro
December 16th 2024LCGC International sat down with Giorgia Purcaro of the University of Liege to discuss the impact that solid-phase microextraction (SPME) and comprehensive multidimensional gas chromatography (GC×GC) is having on food analysis.









