Advances in Microsampling for In Vivo Pharmacokinetic Studies
The Column
Advances in Microsampling for In Vivo Pharmacokinetic Studies in Rodents
Pharmacokinetic (PK) studies are performed throughout the drug discovery process. In general, 250 µL of whole blood is retrieved at each time point, processed to plasma, and stored at -80 °C prior to bioanalysis. Microsampling with dried blood spots (DBS) is an attractive alternative to the conventional whole blood plasma workup. DBS reduces the need to collect large volumes of blood and therefore the number of animals required. However, DBS technology has not been fully embraced as a result of its well-documented hematocrit bias and the labour-intensive sample manipulation required. A new approach - volumetric absorptive microsampling (VAMS) - is gaining traction because it delivers the benefits of DBS and overcomes its limitations while generating comparable PK data to conventional sampling methods.
Pharmacokinetic (PK) studies are routinely performed throughout the drug discovery process, from screening during the lead generation phase to comprehensive PK studies in candidate selection. During hit and lead generation, compounds are synthesized at the milligram level for intravenous (IV) and oral (PO) dosing in a rat or mouse (for example 1 mg/kg for IV and 5 mg/kg for PO). Blood samples are typically taken over a defined time course and three animals are used to obtain an average drug exposure at each time point. A typical study design for discovery PK is shown in Table 1. In general, for rodent PK studies, 250 µL of whole blood is retrieved at each time point, processed to plasma (yielding approximately 100 µL), and stored at -80 °C prior to bioanalysis. Liquid chromatography coupled to tandem mass spectrometry (LC–MS–MS) bioanalysis is performed to assess the pharmacokinetic properties of the molecules and their potential as lead candidates. In general, compounds exhibiting >20% oral bioavailability, moderate to low clearance, and moderate to long terminal halfâlife are prioritized for further profiling in pharmacodynamics (PD) and efficacy studies. With a plethora of highâsensitivity LC–MS–MS systems available today, blood volume is no longer a critical determinant of bioanalytical success, especially for discovery PK studies. Despite these analytical advances, the “one mouse, one time point” study design described above remains common practice.
The Promise of Dried Blood Spotting
Microsampling and, more specifically, the dried blood spot (DBS) technique have gained considerable attention as an alternative to the conventional whole-blood plasma workup used for PK analysis. There are several compelling reasons for the interest in DBS. First, with improvements in LC–MS–MS technology, the blood volume requirements for bioanalysis are significantly smaller. It is somewhat ironic that in the last 10 years, the sensitivity of triple quadrupole mass spectrometers (the “gold standard” for PK bioanalysis) has increased 100× to 1000× relative to earlier generation instruments while blood sampling protocols have stayed the same for many in vivo DMPK and toxicology groups. Second, DBS, relative to plasma, offers a simplified and cost-effective sample collection, storage, and shipping process. Third, and perhaps most importantly, DBS offers the very real opportunity to reduce the number of animals required for PK, toxicokinetic (TK), PD, and efficacy studies. For example, many groups still perform the conventional “one mouse, one time point” PK study design. As shown in Table 1, for a standard mouse PK study design, a total of 27 mice are required for each dosing group. Microsampling blood collection enables the use of far fewer animals because multiple blood samples can be collected from the same animal. The use of fewer animals also eliminates inter-animal variability and is therefore likely to produce more consistent data. For PD and efficacy studies, PK data generally comes from satellite PK groups. This is because the traditional larger volume blood collections preclude the ability to acquire this information from the main study animals. The fact remains that large volumes of whole blood continue to be used out of habit rather than out of necessity and this has translated into the use and sacrifice of more animals than necessary.
With the clear benefits of DBS and microsampling, the legitimate question to ask is why haven’t more groups in drug discovery fully embraced the technology? There are several reasons for this. One of the major reasons is the well-documented hematocrit bias of DBS cards. The blood hematocrit (HCT), or the volume percentage of the whole blood that is comprised of red blood cells, influences the viscosity of blood, and therefore dramatically influences the extent to which a given volume of blood spreads out onto a DBS card. HighâHCT blood will be viscous and tend to not spread out across the card, while low-HCT blood will be more fluid and will spread out farther. Therefore, a given volume of blood will generate different diameter spots on the DBS card depending on its HCT. In a typical DBS workflow a small circular punch is removed from the overall spot for analysis. The effective volume contained in this subpunch is not uniform from sample to sample because it is a function of the overall spot size (which is controlled by the HCT of the blood sample).
To reduce this HCT bias, whole-spot collections can be made from a DBS card. This approach provides a uniform volume; however, it is difficult to automate because of the varied spot sizes and the fact that liquid handlers commonly found in laboratories are not amenable to the card format. Another reason for the slow adoption of DBS is that procedures and techniques for preparing animals for microsampling must be developed and routinized through training. In addition, sample manipulation, including card punching, sample extraction, and handling prior to bioanalysis is more labourâintensive when using DBS cards compared to the simple plasma crash approach that is widely adopted and effectively used by discovery groups to generate PK in discovery.
Recently, a novel volumetric absorptive microsampling (VAMS) approach has been developed to simplify the blood collection process and offer other advantages. An inert, porous, hydrophilic material is used to absorb a set volume of blood, which rapidly “wicks” up the volume independent of hematocrit. Extensive trials have shown that over a wide range of HCT values (20% to 70%) there is no HCT bias associated with the 10 µL volume capture.1,2 Another important differentiating feature of this approach to dried matrix microsampling is that it simplifies the post-sample collection handling and sample extraction protocol. Simple sample handling and extraction is critical in the drug discovery environment because time is of the essence when screening compounds. In the following case study we show how microsampling using the VAMS approach can be applied to simplify blood collection.
Case Study: Volumetric Absorptive Microsampling (VAMS)
In the past, because of the HCT bias issue, there were major concerns that a dried matrix sampling technique could not produce data leading to the same decisions as those resulting from a standard plasma PK data set. To investigate if the volumetric absorptive microsampling (VAMS approach could deliver comparable data, PK profiles were generated and compared for acetaminophen following intravenous dosing in mice using a conventional design (standard plasma processing, n = 21 animals) versus a dried whole-blood design (VAMS microsampling, n = 3 animals for entire time course).
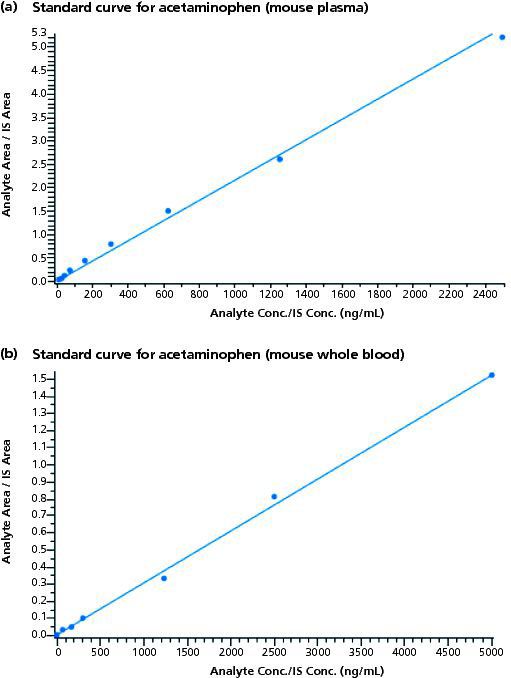
Method: Acetaminophen was formulated in saline to a concentration of 5 mg/mL and a 2 mg/kg dose was administered into the tail vein of the mouse. In group 1, three animals were sacrificed at each time point. A total of 21 mice were required for the entire time course (predose, 0.08 h, 0.25 h, 0.5 h, 1 h, 2 h, and 4 h). Time points past 4 h were not necessary because of the short half-life of acetaminophen in mice. Group 2 animals received acetaminophen through the same route of administration. However, just three mice were used in this study, with each animal bled at every time point across the entire time course. For group 1, at the specific time point, blood was harvested by cardiac puncture. A Mitra microsampling device (Neoteryx LLC) using VAMS technology was dipped into the collected blood and set aside to dry. The remaining blood was processed to plasma and stored at -80 °C until analysis. LC–MS–MS analysis was performed on a Symbiosis HPLC (Spark Holland) coupled to an API4000 QTRAP triple quadrupole ion trap mass spectrometer (Sciex). Chromatographic separations were performed using a 2 mm × 50 mm, 5-μm Kinetex C18 column (Phenomenex). D4-acetaminophen was used as the internal standard. Mobile phase A was water containing 0.1% formic acid. Mobile phase B was acetonitrile containing 0.1% formic acid. The gradient was 1% B to 70% B in 2.5 min following an initial hold at 1% B for 0.5 min. For group 2, blood was retrieved at the specific time point by saphenous vein sampling. Plasma and dried whole-blood standard curves were generated for acetaminophen over the concentration range of 5–5000 ng/mL, as shown in Figure 1. The r-value for the mouse plasma and mouse whole blood was 0.9975 and 0.9986, respectively. These standard curves were then used to quantify the plasma and whole blood exposures, respectively, for acetaminophen following 2-mg/kg intravenous dosing.
Figure 1: Standard curves for acetaminophen: Standards met the criteria of the analytical assay, all measured to within +/- 30% of their target values. R values for plasma and whole blood were 0.9975 and 0.9986, respectively. Standard curves were generated at the front-end and back-end of the study samples.
Shown in Table 2 are the PK parameters for group 1 plasma (conventional) and dried whole blood (VAMS). The area under the curve (AUC), systemic clearance (CLs), and volume of distribution (Vd) were comparable. Only the terminal (elimination) half-life was significantly different (T½ = 0.6 h for plasma versus 0.2 h for dried whole blood). Importantly, in the context of drug discovery, the interpretation of data would be the same - that is, the compound exhibits high clearance, high volume of distribution, and short half-life in the mouse. Given the concordance between the mouse plasma and mouse whole-blood pharmacokinetics incorporating a conventional “one mouse, one time point” paradigm, a group 2 study was performed. For group 2, the pharmacokinetics of acetaminophen using only three mice was evaluated by doing serial bleeding via saphenous vein sampling directly onto the microsampling devices.
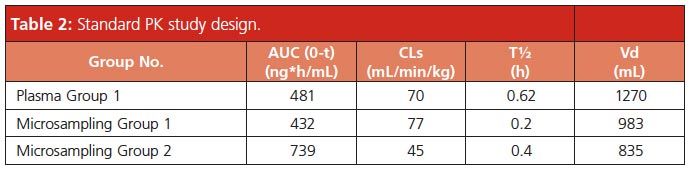
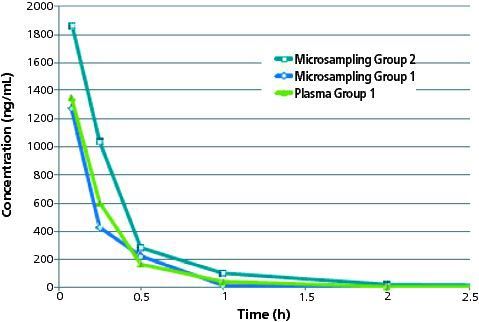
Shown in Figure 2 is the VAMS microsampling whole blood versus time profile for acetaminophen incorporating an n = 3 study design as compared to group 1. The mouse group 2 dried wholeâblood concentration versus time profile was similar to the group 1 result, the primary difference being the measured concentration at the 0.08 h and 0.25 h time points. The acetaminophen dried wholeâblood concentration at t = 0.08 h was 1830 ng/mL, and at t = 0.25 h was 1000 ng/mL. In the case of group 1 animals, the concentration of acetaminophen at the t = 0.08 h time point was 1320 ng/mL. The 0.5 h, 1 h, and 2 h time points showed similar wholeâblood concentrations between the two groups. The differences are likely not attributable to the bioanalytical assay; rather, they may more likely be explained by the source of whole-blood retrieval (saphenous vein versus cardiac puncture) and/or the fact that the group 2 study was conducted on a different day with a different (and fresh) preparation of test article. The PK parameters for group 2 are shown in Table 2. Consistent with the higher exposure at the early time points, the AUC was higher relative to group 1 and the clearance lower. Importantly, the conclusions from this group 2 are consistent with group 1 - that is, the compound exhibits moderate to high clearance, high volume of distribution, short half-life and moderate exposure after intravenous dosing of acetaminophen at 2 mg/kg.
Figure 2: Overlay of concentration versus time profile for microsampling group 1 (cardiac puncture, whole blood wicked onto device tips) and 2 (saphenous vein whole blood wicked onto device tips) and plasma group 1 (cardiac puncture, whole blood processed to plasma). The 5 min (0.08 h) microsampling group 2 time point shows slightly higher concentration than plasma and whole blood for study 1 animals.
Conclusion
The data presented in this article suggest that volumetric absorptive microsampling technology is a very useful collection technique for pharmacokinetic studies involving mice, greatly reducing the animal number requirement and opening the door to the possibility of combining PK and PD and efficacy assessments in the same study animals. This advance in microsampling could quite possibly change the “one mouse, one time point” paradigm commonly found in early discovery pharmaceutical development. Ultimately, pharmaceutical companies will need to decide whether the potential differences in quantitative bioanalytical data are truly meaningful and might present any legitimate risk to the drug discovery and development process as well as if those differences outweigh the benefits that dried matrix microsampling provide.
References
1. Neil Spooner, Philip Denniff, Luc Michielsen, et al., Bioanalysis 7(6), 653–659 (2015).
2. Philip Dennif and Neil Spooner, Anal. Chem.86(16), 8489–8495 (2014).
Stuart Kushon currently holds the position of senior research scientist at Neoteryx LLC, a company dedicated to developing novel microsampling solutions for the pharmaceutical and clinical markets. Kushon is a physical organic chemist with over 10 years of experience developing products for the direct detection and analysis of targets including: viral, bacterial, and protein pathogens as well as small molecules that serve as diagnostic markers for disease states.
Daniel Kassel founded SciAnalytical Strategies in 2013, a bioanalytical, biomarker discovery, and consulting firm that serves the pharmaceutical, biotechnology, and clinical industries. Kassel also maintains a half-time appointment as director of research and development for InSource Diagnostics, a research-driven clinical diagnostics organization.
Hong Xin is a biotechnology entrepreneur specializing in preclinical research with extensive experience in contract research and operations, with academic knowledge across multiple disciplines including cancer biology, cell biology, molecular biology and pharmacology. Xin currently works at Explora BioLabs as COO.
Nurith Amitai is a biomedical scientist with 10 years of predoctoral and postdoctoral training, who previously held the position of Scientist I at Explora BioLabs in San Diego, California, USA.
E-mail: cathyc@neoteryx.comWebsite: www.neoteryx.com
This article was featured in LCGC's digital magazine The Column. Click here to view the full issue>>

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.




