In-Well Hydrolysis Using UCT’s Refine Ultra-Filtration Technology
UCT's Refine™ Ultra-Filtration technology allows for sample precipitation and filtration to occur simultaneously in the individual column/well. A novel, hydrophobically treated, submicron frit combination facilitates the removal of sample proteins without a complicated extraction.

Procedure
Sample Extraction
a) Place a collection plate under the Refine™ Ultra-Filtration Plate.
b) Add 250 µL of urine and 250 µL Abalonase Ultra Enzyme working solution.
c) Hydrolyze for 30 min at 55 °C.
d) Further dilute sample by adding 500 µL D.I. H2O.
e) Mix sample. This can be executed via vortexing at maximum speed or multiple pipette aspirations and dispenses.
f) Filter the sample using one of the following techniques:
1. Centrifuge: For 5 min at 500 g or until filtrate is collected.
2. Vacuum: Apply vacuum at ~20" of Hg for up to 5 min or until filtrate is collected.
3. Positive Pressure: Apply 2–5 psi using positive pressure for up to 5 min or until filtrate is collected.
Instrumental
LC–MS/MS: Agilent™ 1200 HPLC and AB Sciex™ 4000 Q Trap (MS/MS)
Column: UCT Selectra® DA HPLC column 100 × 2.1 mm, 3 µm
Guard Column: UCT Selectra® DA guard column 10 × 2.1 mm, 3 µm
Injection volume: 10 µL
Mobile phase A: D.I. H2O + 0.1% formic acid
Mobile phase B: MeOH + 0.1% formic acid
Column flow rate: 0.30 mL/min
Results
Table I: Percent reduction in analyte suppression following use of Refine™ plate compared to dilute and shoot preparation for hydrolyzed urine samples.
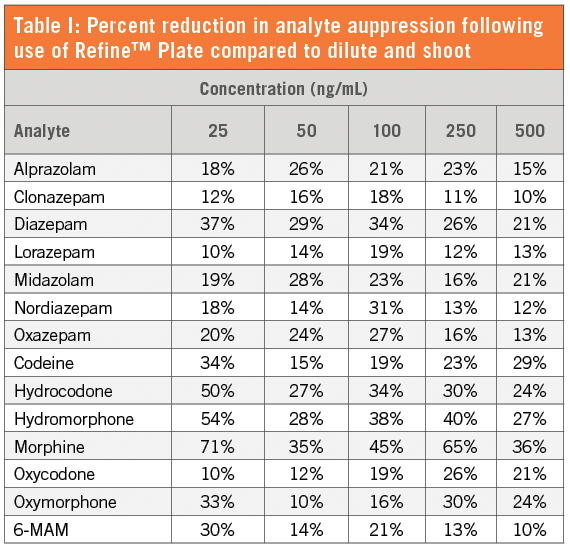
Conclusion
Using the above procedure, the Refine™ Ultra-Filtration technology allowed for a reduction in instrumental back pressure and removal of unwanted matrix components in urine samples. Overall, this allows for an end user to have enhanced analyte selectivity and an increase in HPLC/UPLC column life.
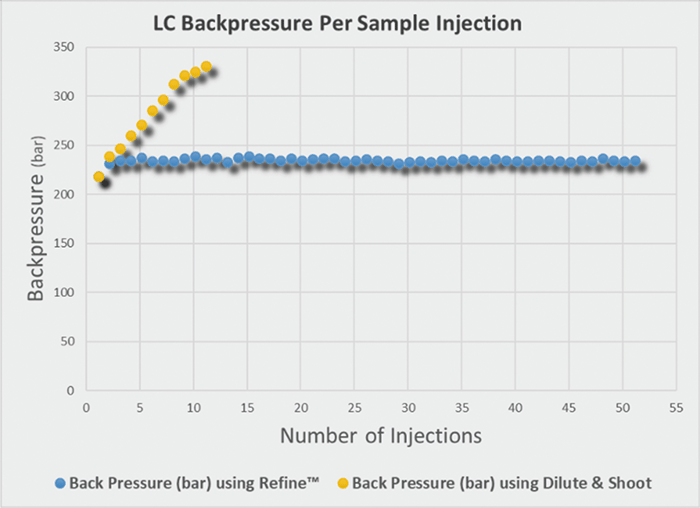
Figure 1: Back pressure per sample injection of Refine™ Ultra Filtration technology versus a dilute and shoot preparation for hydrolyzed urine samples.

UCT, LLC
2731 Bartram Rd, Bristol, PA 19007
tel: (800) 385-3153
Website:www.unitedchem.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








