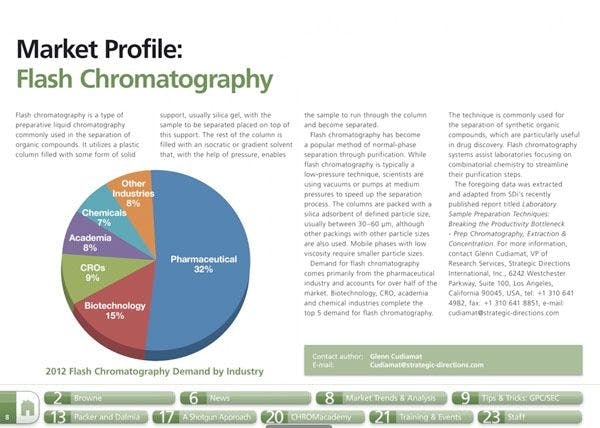
Vitamin D supplements' potency varies widely
The amount of vitamin D in supplements can be anything from 9% to 146% of the stated dose, according to a research letter published in the JAMA Internal Medicine.1

The amount of vitamin D in supplements can be anything from 9% to 146% of the stated dose, according to a research letter published in the JAMA Internal Medicine.1 Investigators from Kaiser Permanente (Portland, Oregon, USA) tested 55 bottles of over-the-counter vitamin D supplement tablets from 12 different manufacturers using high performance liquid chromatography (HPLC).
Vitamin D supplements are often prescribed for vitamin D deficiencies, but are not regulated by the Food and Drug Administration (FDA). There are a range of reports suggesting that a vitamin D deficiency may have links to a number of health problems from neurological disorders to hypertension, as reported in The Column2 last year. Hypovitaminosis D, a deficiency of vitamin D from either dietary or sunlight sources, is now widely recognized to be an affliction of the western world as a result of low sunlight exposure and poor diet.
Supplement tablets purchased from Portland pharmacies were analysed by HPLC and found to contain 9–146% of the labeled concentration. This variation was found not only between tablets produced by manufacturers but also between tablets contained within the same bottle.
“We were surprised by the variation in potency among these vitamin D pills,” said study co-author Erin LeBlanc, lead author and investigator, Kaiser Permanente Center for Health Research (Portland, Oregon, USA). “The biggest worry is for someone who has low levels of vitamin D in their blood. If they are consistently taking a supplement with little vitamin D in it, they could face health risks.”
Manufacturers can voluntarily submit supplements to accreditation by US Pharmacopeial Standards (USP). These standards require tablets to contain between 90% and 110% active ingredient. According to the report, these may be sparsely distributed.
“The USP verification mark may give consumers some reassurance that the amount of vitamin D in those pills is close to the amount listed on the label”, commented LeBlanc. “There are not many manufacturers that have the USP mark, but it may be worth theextra effort to look for it.”
References
1. E.S. LeBlanc, N. Perrin, J. Johnson Jr, A. Ballatore and T. Hiller, JAMA Intern. Med., DOI: 10.1001/jamainternmed.2013.3812 (2013).
2. Vitamin D detection, The Column 20(8) 6 (2012) http://www.chromatographyonline.com/lcgc/article/articleDetail.jsp?id=796682&sk=ea1ac8c2df2bd242b00772da76a994d8
This story originally appeared in The Column. Click here to view that issue.

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.