Using pH-LC to Control Selectivity of Acidic and Basic Compounds on Gemini-NX
The Application Notebook
The use of mobile phase pH to control analyte ionization states (pH-LCâ„¢) in reversed phase HPLC separations is a highly effective way to change selectivity. The ionized species of an analyte is shown to have higher polarity (less hydrophobicity) than the neutral species, which results in a loss of expected retention for that analyte. This can be attributed to less interaction with the hydrophobic stationary phase and greater affinity with the aqueous portion of the mobile phase. Ionized species also participate in ionic interactions with exposed and activated silanols, which impact peak shape and reproducibility.
The use of mobile phase pH to control analyte ionization states (pH-LC™ ) in reversed phase HPLC separations is a highly effective way to change selectivity. The ionized species of an analyte is shown to have higher polarity (less hydrophobicity) than the neutral species, which results in a loss of expected retention for that analyte. This can be attributed to less interaction with the hydrophobic stationary phase and greater affinity with the aqueous portion of the mobile phase. Ionized species also participate in ionic interactions with exposed and activated silanols, which impact peak shape and reproducibility.
Acidifying the mobile phase to neutralize silanol groups is a common approach to reduce secondary interactions. However, this does not resolve the issue of ionized analytes having less hydrophobic interaction with the stationary phase, especially basic compounds with a high pKa. In addition, all surface silanol groups may not share the same pKa and therefore are not all neutralized at the same pH.
The relationship between mobile phase pH and analyte ionization states is shown in Figure 1.
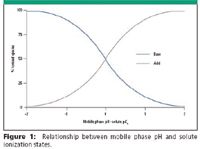
Figure 1
In this figure, the mobile phase pH in which there is partial dissociation (mixture of ionized and neutral species) of acids and bases, occurs within two pH units above and below the analyte pKa. In order to have predominantly single ionization states in solution, the mobile phase pH must be at least two units above the analyte pKa.
We investigated the effectiveness of using pH-LC™ techniques in the separation of a mixture of acidic (naproxen), basic (amitriptyline), and neutral (toluene) compounds using a Gemini® -NX 5 μm C18, 150 × 4.6 mm, which was designed to be very tolerant of changes in mobile phase pH without column degradation. The structures and pKa are shown in Table I.
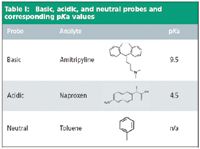
Table I: Basic, acidic, and neutral probes and corresponding pKa values
Experimental Conditions
System
Agilent HP1100 HPLC system equipped with quaternary pump (G1311A), degasser (G1322A), autosampler (G1313A) and DAD detector (G1315B). A heating/cooling, 6-column selector (POWERSelector™ ) by Analytical Sales and Services, Inc was used. Data analysis using ChemStation Software (Rev A.10.02).
Materials
Formic acid, 98 % ACS Grade, by EMD (FX0440-11)
Ammonium acetate, HPLC grade, by Fluka (17836)
Potassium phosphate, monobasic, ACS grade, by Fluka (P285-
3)
Ammonium bicarbonate, >99 %, by Fluka (09832)
Amitriptyline hydrochloride, >98 %, by Sigma (A8404)
Naproxen, 98 %, by Aldrich (284785)
Toluene, 99.5 %, by Sigma (179418)
Conditions
a) pH 2.7 (App ID 16593)
Mobile Phase: A: 0.1 % Formic Acid in Water
B: 0.1 % Formic Acid in Acetonitrile
Gradient: A/B (95:5) to (5:95) in 10 min, Hold for 2 min
Flow Rate: 1.5 mL/min
Temperature: Ambient
Detection: UV @ 254 nm
Sample:
1. Amitriptyline
2. Naproxen
3. Toluene
b) pH 4.8 (App ID 16598)
Mobile Phase: A: 10 mM Ammonium Acetate pH 4.8
B: Acetonitrile
Gradient: A/B (95:5) to (5:95) in 10 min, Hold for 2 min
Flow Rate: 1.5 mL/min
Temperature: Ambient
Detection: UV @ 254 nm
Sample:
1. Amitriptyline
2. Naproxen
3. Toluene
c) pH 7.0 (App ID 16600)
Mobile Phase: A: 20 mM Potassium Phosphate pH 7.0
B: Acetonitrile
Gradient: A/B (95:5) to (5:95) in 10 min, Hold for 2 min
Flow Rate: 1.5 mL/min
Temperature: Ambient
Detection: UV @ 254 nm
Sample:
1. Amitriptyline
2. Naproxen
3. Toluene
d) pH 10.5 (App ID 16601)
Mobile Phase: A: 10 mM Ammonium Bicarbonate pH 10.5
B: Acetonitrile
Gradient: A/B (95:5) to (5:95) in 10 min, Hold for 2 min
Flow Rate: 1.5 mL/min
Temperature: Ambient
Detection: UV @ 254 nm
Sample:
1. Amitriptyline
2. Naproxen
3. Toluene
Results and Discussion
This work demonstrated the different effects of mobile phase pH using various buffers on a mixture of an acid, base, and neutral probes.
Figure 2a: At mobile phase pH 2.7, the base amitriptyline (pKa 9.4) is ionized and exhibits retention at 5.2 min. The acid naproxen (pKa 4.5) is neutral and exhibits retention at 7.7 min. The neutral probe, toluene, will show relatively unchanged retention (8.7 min) throughout the pH range.
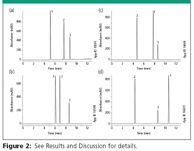
Figure 2
Figure 2b: At mobile phase pH 4.8 the base amitriptyline shows increased retention (6.1 min), which may be attributed to ionic interaction with activated silanols. The acid naproxen is partially dissociated (ionized and neutral) and shows a loss of retention (7.0 min). The retention of the neutral compound toluene remains unchanged.
Figure 2c: At mobile phase pH 7.0, the base amitriptyline shows a slight increase in retention (7.8 min) while the acid naproxen, which is approaching a predominantly ionized species, drops in retention (4.8 min).
Figure 2d: At mobile phase pH 10.5, the base amitriptyline is predominantly neutral and exhibits the most retention (10.6 min), almost double compared to retention at pH 2.7. The acid naproxen shows minimal decrease in retention (4.3 min) as it remains in a predominantly ionized state. Toluene has remained unchanged.
Conclusion
With the use of mobile phase pH in conjunction with the pH stable column, Gemini-NX C18, powerful selectivity manipulation can be achieved when working with ionizable compounds in reversed phase HPLC. Increased retention for most basic compounds can be achieved by increasing the mobile phase pH above their pKa values. The same benefits can be achieved for most acidic compounds by decreasing the mobile phase pH below their pKa values.
The method flexibility and improved selectivity provided with these techniques make Gemini-NX ideal for new method development where the columns may be exposed to a variety of pH conditions before optimized compound-dependent methods are established.

Phenomenex, Inc.
411 Madrid Ave.
Torrance, CA 90501
(310) 212-0555; (310) 328-7768

Determination of 3-MCPD and Glycidol in oil by ISO 18363-1, AOCS Cd 29c-13, DGF C-VI 18 (10)
January 28th 2025Fully automated method for 3-MCPD and Glycidol determination in edible oil by GC-MS, based on the widely used methods ISO 18363-1, AOCS Cd 29c-13, and DGF C-VI 18 (10). The automated GC-MS determination of 3-MCPD and glycidol in edible oils with evaporation step and GC column backflush ensures low LODs by eliminating excess derivatization reagent for improved method stability and system ruggedness.
Determination of 3-MCPD, 2-MCPD and Glycidol in oil and fat by ISO 18363-4 Zwagerman/Overman
January 28th 2025Fully automated method for 3-MCPD, 2-MCPD and Glycidol determination in Edible Oil and Fat based on ISO 18363-4 - Zwagerman/Overman with validation data. A recent upgrade to PTV injection has further improved the quality and robustness of results. Fatty acid esters of 3- and 2-monochloropropanediol (3-MCPD-e, 2-MCPD-e) and glycidol (Gly-e) are process contaminants that are formed, for example, when edible oils and fats are refined. After ester cleavage during digestion in the human body they pose a relevant health risk and therefore need to be determined in edible oils and fats and in fat containing food.
Automated Analysis of MOSH/MOAH in food and packaging extracts by LC-GC-FID
January 28th 2025The AppNote describes the fully automated determination of MOSH/MOAH in Food and Packaging extracts following DIN EN 16995. Industrial production, processing and transportation invariably put food at risk of contamination with MOSH/MOAH. To ensure a reasonable cost benefit balance, high laboratory productivity and good quality of results, leading contract laboratories increasingly strive to automate their processes. An example is the determination of MOSH/MOAH using a LC-GC-FID Coupling Platform. Depending on the sample matrix, additional automated sample preparation by aluminum oxide clean-up, epoxidation, and/or saponification is necessary prior to analysis. The dedicated evaluation software integrates the complex MOSH/MOAH chromatograms accurately and reproducibly.
Testing Solutions for Metals and PFAS in Water
January 22nd 2025When it comes to water analysis, it can be challenging for labs to keep up with ever-changing testing regulations while also executing time-efficient, accurate, and risk-mitigating workflows. To ensure the safety of our water, there are a host of national and international regulators such as the US Environmental Protection Agency (EPA), World Health Organization (WHO), and the European Union (EU) that demand stringent testing methods for drinking water and wastewater. Those methods often call for fast implementation and lengthy processes, as well as high sensitivity and reliable instrumentation. This paper explains how your ICP-MS, ICP-OES, and LC-MS-MS workflows can be optimized for compliance with the latest requirements for water testing set by regulations like US EPA methods 200.8, 6010, 6020, and 537.1, along with ISO 17294-2. It will discuss the challenges faced by regulatory labs to meet requirements and present field-proven tips and tricks for simplified implementation and maximized uptime.
A Guide To Finding the Ideal Syringe and Needle
January 20th 2025Hamilton has produced a series of reference guides to assist science professionals in finding the best-suited products and configurations for their applications. The Syringe and Needle Reference Guide provides detailed information on Hamilton Company’s full portfolio of syringes and needles. Everything from cleaning and preventative maintenance to individual part numbers are available for review. It also includes selection charts to help you choose between syringe terminations like cemented needles and luer tips.