Use of Amine Additive in the Sample Diluent in a Supercritical Fluid Chromatography (SFC) Purification
Basic compounds often require basic additives in the modifier to improve peak shape and resolution in SFC (1). These additives can be difficult to remove post-purification (2).
Basic compounds often require basic additives in the modifier to improve peak shape and resolution in SFC (1). These additives can be difficult to remove post-purification (2). In this application note, we present a chiral SFC purification of a proprietary AstraZeneca zwitterionic compound, containing a functionalized pyrimidone and other aromatic substituents, known to retain amine additives after isolation. Dimethylethylamine was added to the sample diluent to improve the chromatography and to avoid further purification of the desired stereoisomer to remove the amine.
Experimental Conditions
SFC System: Thar MultiGram III Preparative SFC
Column: Chiralpak AD-H, 5 µm, 30 × 250 mm
Modifier: Isopropanol
Mobile phase: 75% Carbon dioxide, 25% isopropanol
Flow rate: 120 mL/min
Detection: UV at 254 nm
Column temperature: 40 °C
Outlet pressure: 100 bar
Results
Figure 1 shows the chiral separation of an AZ compound in neutral modifier and sample diluent. Three peaks eluted off the semi-preparative column. The first peak contained a mixture of both stereoisomers. The second peak was primarily Stereoisomer 1, while the third peak was pure Stereoisomer 2. Previous batches of this compound were purified by high performance liquid chromatography (HPLC) using an amine additive in the modifier, and the NMR data showed that the isolated stereoisomers contained the amine after dry-down.

Figure 1: Semi-preparative chromatogram, 64 mg injection (loading: 4 mL of a 16 mg/mL sample solution). The sample diluent and the modifier were neutral.
Figure 2 shows the same separation with the addition of one percent of dimethylethylamine, by volume, to the sample diluent instead of the modifier. The amine eluted at the beginning of the run and the two stereoisomers were well-resolved.
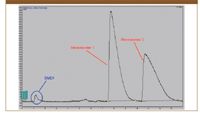
Figure 2: Semi-preparative chromatogram, 128 mg injection (loading: 8 mL of a 16 mg/mL sample solution). Dimethylethylamine (1% by volume) was added to the sample diluent. The modifier was neutral.
Since Stereoisomer 2 was the desired compound, the stacking conditions were programmed to collect dimethylethylamine with Stereoisomer 1. Stereoisomer 2 was isolated and did not contain dimethylethlamine after dry-down.
Conclusions
The addition of dimethylethylamine to the sample diluent improved chromatography, and the lack of the amine in the modifier ensured its absence in the desired fraction. This eliminated the need for a subsequent purification for amine removal.
References
(1) M. Maftouh, C. Granier-Loyaux, E. Chavana, J. Marini, A. Pradines, Y.V. Heyden, and C. Picard, J. Chrom. A 1088, 67–81 (2005).
(2) C. Hamman, D.E. Schmidt Jr., M. Wong, and M. Hayes, J. Chrom. A 1218, 7886–7894 (2011).

Follow the Data to Grow: Why a Scientific Data Platform is Essential
October 28th 2024Innovation is the engine that powers a company’s growth and product development, and for enterprises with R&D laboratories, those lab environments are the greatest source of this innovation. In this white paper, learn how a platform approach to scientific data management, including semantic search, advanced analytics, and lab automation, leads to better enterprise decisions at the executive level, optimized lab performance, more discoveries, and stronger product pipelines.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)















