An Ultrahigh-Performance Liquid Chromatography Alternative to Animal Testing for Skin Sensitization
The amino acid derivative reactivity assay (ADRA) is an innovative alternative test method for skin sensitization without animal abuse that was adopted by the Organization for Economic Cooperation and Development (OECD) in 2019. This article describes OECD-compliant ADRA testing and method transfer to ultrahigh-performance liquid chromatography (UHPLC) conditions to reduce analysis time from 20 min to 7 min.
Skin sensitization tests evaluate the allergic reactivity of skin to chemical substances. For animal welfare, alternative methods are being developed for these tests. Chemical substances can induce an inflammatory reaction if they bind to cysteine or lysine in epidermal proteins (1,2). International test guidelines for evaluating chemical substances, published by the Organization for Economic Cooperation and Development (OECD) (3), include the direct peptide reactivity assay (DPRA) and amino acid derivative reactivity assay (ADRA) as alternative in chemico test methods based on the properties indicated above. ADRA evaluates the binding characteristics of test substances using N-(2-(1-naphthyl)acetyl)-L-cysteine (NAC) or α-N-(2-(1-naphthyl)acetyl)-L-lysine (NAL), which are synthesized by inducing cysteine or lysine, respectively, to bind with naphthalene rings. ADRA sample solutions are analyzed at 1/100 of the concentration analyzed for DPRA. The presence of naphthalene rings enables detection at a 281-nm UV wavelength, where effects from any detection of contaminants are minimal. The ADRA test method and recommended chemicals for demonstrating technical proficiency are specified in OECD TG 442C Appendix II. Proficiency tests are performed to assess the technical proficiency of testing personnel and the given testing environment in terms of the binding characteristics of NAC and NAL (NAC and NAL percent depletion) with respect to any of the eight substances from the assigned 10 compounds. In this study, the system suitability and proficiency tests described were all performed according to the conditions specified by the OECD before the methodology was adapted to ultrahigh‑performance liquid chromatography (UHPLC) conditions. Apart from the shorter analysis time, there was no difference in the results obtained with the optimized conditions.
ADRA Testing by HPLC
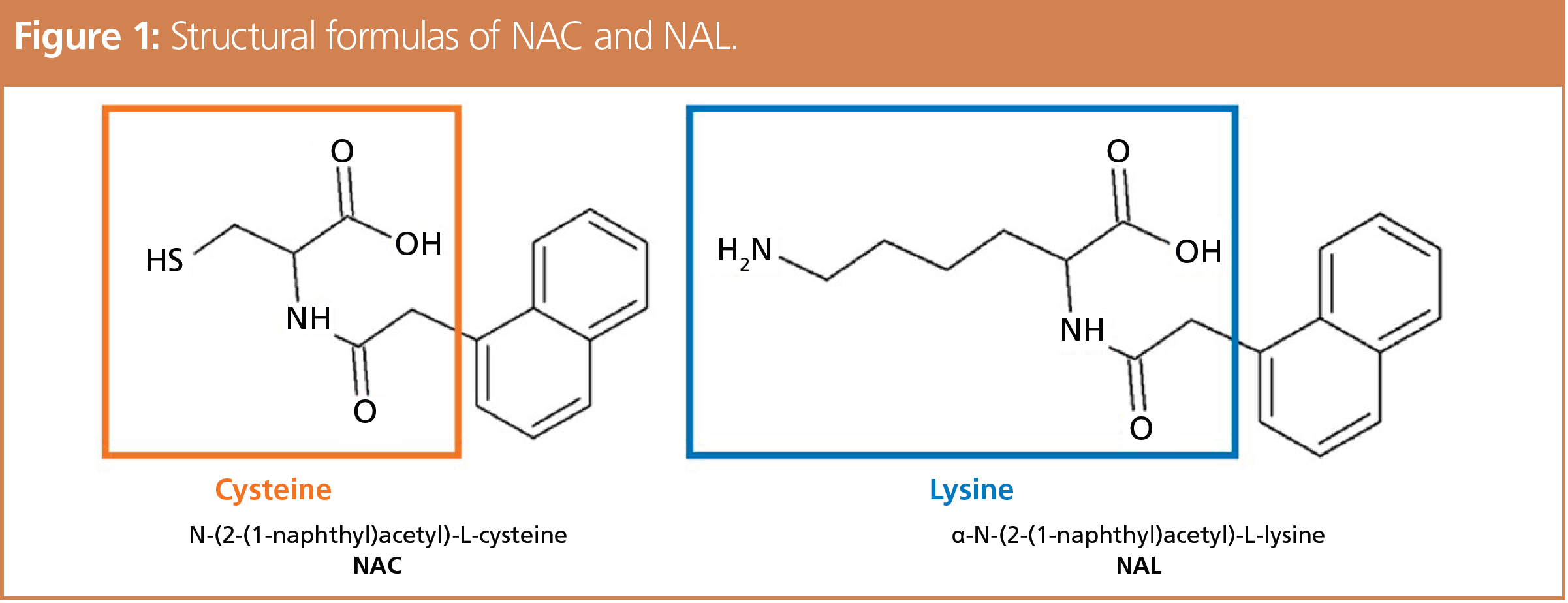
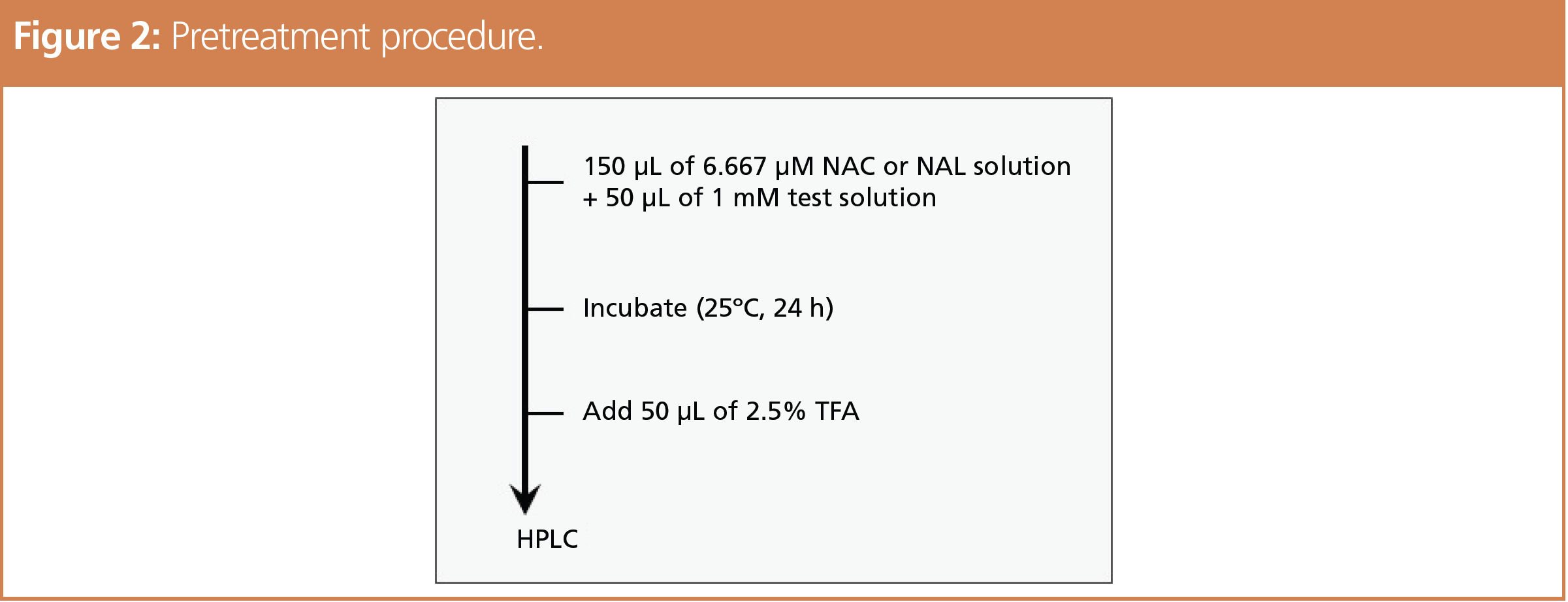
The structural formulas of NAC and NAL used to evaluate the protein reactivity by ADRA are shown in Figure 1. After allowing the test substance solution to react with the NAC and NAL solutions, the tendency of the test substances to bind with cysteine and lysine was evaluated by using high performance liquid chromatography (HPLC) to measure the percent depletion of NAC or NAL. The analytical conditions are indicated below and the pretreatment procedure in Figure 2. The reagents were prepared using an ADRA kit (Fujifilm Wako Pure Chemical).
Analytical Conditions of ADRA Testing by HPLC:
System: Nexera XR UHPLC system (Shimadzu); column: 150 mm × 3.0 mm, 2.7-μm Shim-pack Velox C18 (Shimadzu); flow rate: 0.3 mL/min; mobile phase: 0.1% TFA in A) water and B) acetonitrile; time program for NAC: 30% B (0 min) → 55% B (9.5 min) → 100% B (10–13 min) → 30% B (13.5–20.00 min); time program for NAL: 20% B (0 min) → 45% B (9.5 min) → 100% B (10–13 min) → 20% B (13.5–20.00 min); column temp.: 40 ˚C; injection volume: 10 μL; detection (PDA); λ = 281 nm.
System Suitability and Proficiency Testing
Seven-point calibration curves were prepared for NAC and NAL from 0 (blank) to 5 μM, which indicated good linearity, with an r2 value > 0.999. Subsequent test results for reference control samples to verify that NAC and NAL concentrations were within the specified range and ensure system stability met all acceptance criteria. They also served as a measure of reactivity of the solvent used for NAC, NAL, and each test substance, and as a reference for calculating percent depletion values.
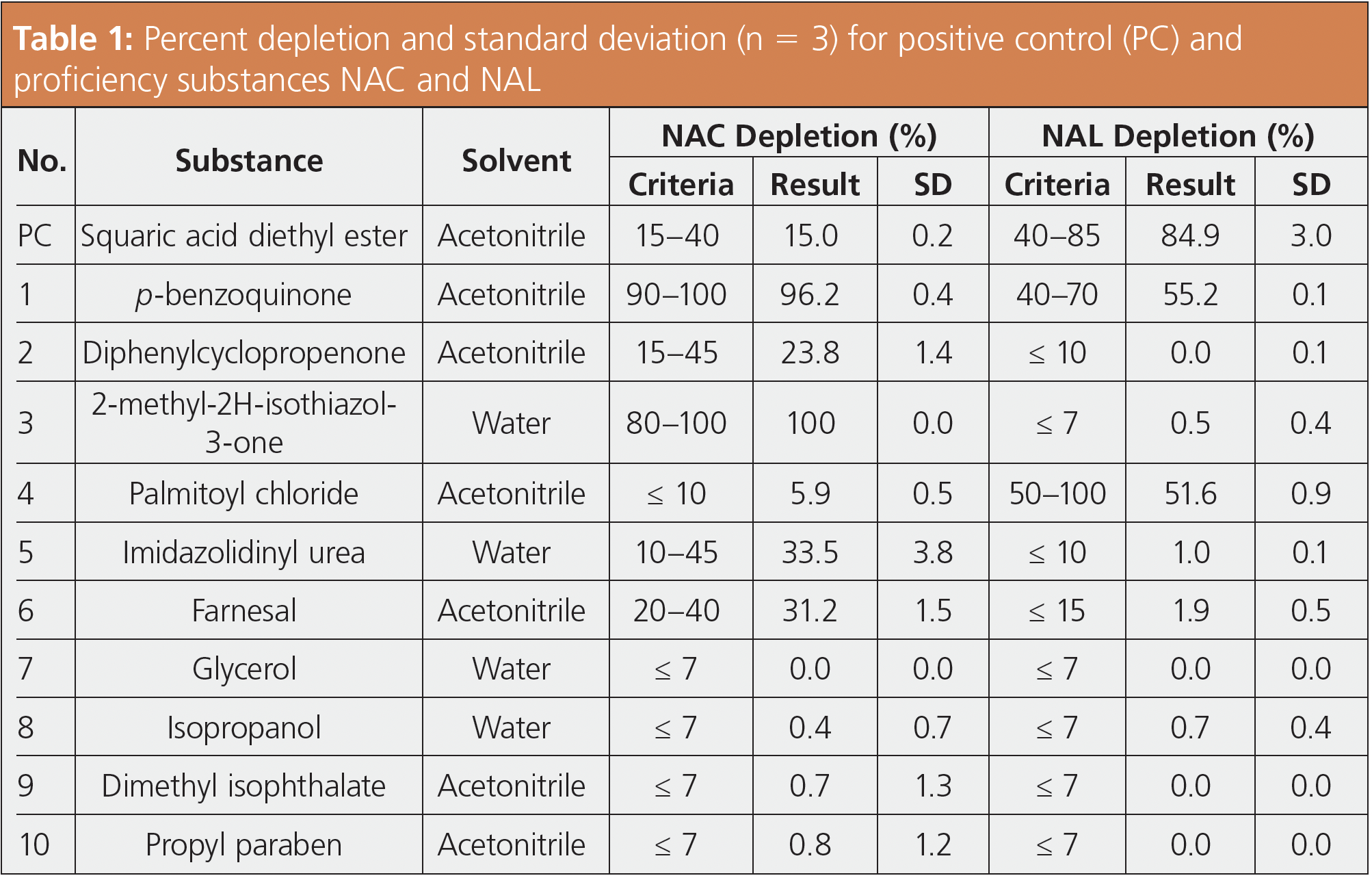
Proficiency was then evaluated for the 10 test substances specified by the OECD. Skin sensitization to test substances was determined based on the percent depletion of NAC and NAL. The formula used to calculate percent depletion values is shown in equation 1. According to the procedure for evaluating technical proficiency, a passing result requires that the percent depletion for both NAC and NAL must be within the specified ranges and the standard deviation (SD) for n = 3 samples 10% or less. For system suitability, the procedure required that reference values were satisfied for at least eight of the 10 proficiency substances. Chromatograms for the positive control (PC) and proficiency substances are shown in Figure 3, with the corresponding depletion rates and standard deviation values listed in Table 1. The results satisfied reference values for all 10 proficiency substances.


Method Optimization
The OECD recommends a 2.7 μm particle column suitable for UHPLC analysis, but the corresponding analytical method specifies standard HPLC conditions that result in an analysis time of about 20 min for each sample. Following this guidance, the time required for evaluation of 10 substances, including system suitability and proficiency tests, would amount to 50 h. To reduce the time for each test, the method was adapted to UHPLC conditions while using the same analytical column, which resulted in a run time of 7 min for each analysis. The optimized analytical conditions are listed below.
Analytical Conditions of ADRA Testing by UHPLC:
System: Nexera XR UHPLC system (Shimadzu); column: 150 mm × 3.0 mm, 2.7-μm Shim-pack Velox C18 (Shimadzu); flow rate: 1.0 mL/ min; mobile phase: 0.1% TFA in A) water and B) acetonitrile; time program for NAC: 30% B (0 min) → 55% B (3.5 min) → 100% B (3.6–5.0 min) → 30% B (5.1–7.0 min); time program for NAL: 20% B (0 min) → 45% B (3.5 min) → 100% B (3.6–5.0 min) → 20% B (5.1–7.0 min); column temp.: 40 ˚C; injection volume: 10 μL; detection (PDA): λ = 281 nm.
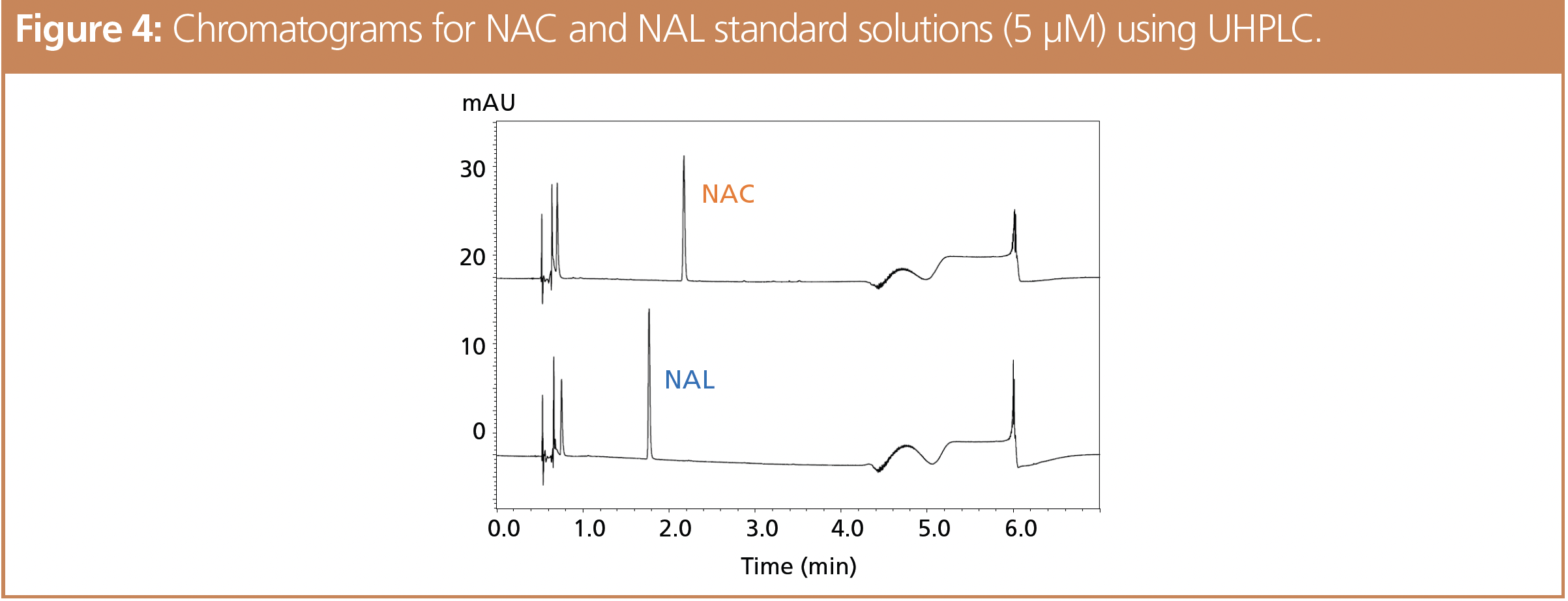
Chromatograms for determination of NAC and NAL in standard solutions using these UHPLC conditions are shown in Figure 4. Calibration curves in the concentration range from 0 (blank) to 5 μM showed good linearity, with r2 values > 0.999 for both compounds. The system suitability results were equivalent to those obtained using HPLC conditions as specified by the OECD.
Conclusion
A fast and simple UHPLC method for ADRA skin sensitization testing was successfully adopted from the HPLC method specified by the OECD. By using an UHPLC system and a C18 column with core–shell technology, the change in separation conditions did not require the use of a different instrument or separation column. System suitability and proficiency tests complied with all specified values, while analysis time could be reduced from the 50 h required for 10 test substances to only 18 h.
References
(1) Hayashida, M.; Nomura, A. Shimadzu Corporation Application News No. 01-00258-EN, Shimadzu Corporation, November 2021. https://www.shimadzu.com/an/sites/shimadzu.com.an/files/pim/pim_document_file/applications/application_note/14427/an_01-00258-en.pdf (accessed 2023-06-13).
(2) Hayashida, M.; Nomura, A. Shimadzu Corporation Application News No. 01-00304-EN, Shimadzu Corporation, September 2022. https://www.shimadzu.com/an/sites/shimadzu.com.an/files/pim/pim_document_file/applications/application_note/16860/an_01-00304-en.pdf (accessed 2023-06-13).
(3) OECD, Guidelines for the Testing of Chemicals: Test No. 442C: In Chemico Skin Sensitisation, OECD iLibrary (2022).
Gesa Johanna Schad graduated with a diploma in chemical engineering from the Technical University NTA in Isny, Germany, in 2004 and a M.Sc. in pharmaceutical analysis from the University of Strathclyde in Glasgow, UK, in 2005. She worked until 2006 as a consultant in HPLC method development and validation in an analytical laboratory of the FAO/IAEA in Vienna, Austria. She earned her doctorate for research in pharmaceutical sciences at the University of Strathclyde in 2010 and was employed as an HPLC specialist in the R&D department at Hichrom Ltd in Reading, UK, from 2009. Since 2013, she has worked as an HPLC product specialist and since 2015 as an HPLC product manager in the analytical business unit of Shimadzu Europa in Duisburg, Germany.
Momoka Hayashida graduated with a diploma in biology from Ochanomizu University in Tokyo, Japan, in 2017 and a master’s degree of life sciences from the Graduate School of Humanities and Sciences, Ochanomizu University in Tokyo in 2019. She has worked as a HPLC product specialist at the Solutions Center of Excellence of Shimadzu Corporation in Kyoto, Japan, since 2019.
Ayako Nomura graduated with a diploma in education from the University of Yamanashi in Yamanashi, Japan, in 1996 and a master’s degree of education from the Graduate School of Education, University of Yamanashi in Yamanashi in 1998. She worked as a sales representative for two years and has worked as an HPLC application specialist for 23 years at Shimadzu Corporation, Japan.

Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)