Ultra-Sensitive Method for the Detection of Illegal Dyes in Food Spices
Synthetic azo- and non-azo dyes were once commonly used as food colourings in many countries. Food safety regulators in Europe, the U.S, and other countries have now banned the use of these synthetic dyes in food because of their potential genotoxic and carcinogenic effects. In some countries, however, these dyes are still being used, especially in spices. There are currently no published legal limits for these illegal food dyes, but any detectable amount is deemed unacceptable. Thus, any analytical method used to test foods for these illegal dyes must be highly sensitive. Conventional methods are only able to provide limits of quantitation (LOQs) of 10–1000 ppb for these illegal food dyes. A reversed-phase ultrahigh-pressure liquid chromatography tandem mass spectrometry (UHPLC–MS/MS) method has been developed that reliably achieves LOQs that are three-to-four orders of magnitude lower than conventional methods while also providing improved accuracy and reproducibility.
Photo Credit: Africa Studios/ Shutterstock.com

Wilhad M. Reuter, PerkinElmer, Inc., Shelton, Connecticut, USA
Synthetic azo- and non-azo dyes were once commonly used as food colourings in many countries. Food safety regulators in Europe, the U.S, and other countries have now banned the use of these synthetic dyes in food because of their potential genotoxic and carcinogenic effects. In some countries, however, these dyes are still being used, especially in spices. There are currently no published legal limits for these illegal food dyes, but any detectable amount is deemed unacceptable. Thus, any analytical method used to test foods for these illegal dyes must be highly sensitive. Conventional methods are only able to provide limits of quantitation (LOQs) of 10–1000 ppb for these illegal food dyes. A reversed-phase ultrahigh-pressure liquid chromatography tandem mass spectrometry (UHPLC–MS/MS) method has been developed that reliably achieves LOQs that are three-to-four orders of magnitude lower than conventional methods while also providing improved accuracy and reproducibility.
Synthetic azo and non-azo dyes are used in numerous nonfood products such as solvents, oils, waxes, plastics, and textiles. Until relatively recently, they were also used as food colourings to enhance or maintain the appearance of certain foodstuffs. It was common industry practice for these synthetic dyes to be used in spices such as paprika, turmeric, various chili powders, curry powders and pastes, and foods containing these spices. Even meats were not exempt from a little visual “spicing up!”
Research findings from the 1990s and early 2000s, in conjunction with earlier data, began to clarify the potential human health risks from exposure to these synthetic dyes. In particular, metabolic studies suggest that the degradation products of these dyes could be genotoxic or carcinogenic (1,2,3,4,5).
Regulatory Status
Based on these findings, food safety regulators in the European Union, United States, China, and several other countries banned the use of these synthetic dyes in food. There are currently no published legal limits for these food dyes, but any detectable amount is deemed unacceptable in nearly all countries. Despite the ban, the illegal dyes are still found in some foodstuffs, particularly spices, whether as a result of accidental cross-contamination or deliberate attempts to enhance the food product (6). Therefore, routine testing of certain foods for these illegal dyes remains a priority to protect human health.
Analytical Challenges
Given the objective of eliminating all traces of the illegal dyes from foodstuffs, any analytical method used must be highly sensitive-and therein lies the recurring problem. Methods used to-date include gas chromatography–mass spectrometry (GC–MS), liquid chromatography (LC)–MS, high performance liquid chromatography–ultraviolet (HPLC–UV), and HPLC–MS. These conventional methods provide limits of quantitation (LOQs) for the target dyes that range from 10 ppb to 1000 ppb-nowhere near the sensitivity needed (6). These methods also commonly encounter matrix interference from the foods being tested that makes it difficult to produce accurate and reproducible data (6). The need has been present for some time for an adequately sensitive, accurate, and reproducible analytical method for testing food samples for the presence of these illegal dyes.
A new reversed-phase UHPLC–MS/MS method for assessing the presence of these illegal dyes in spices has been developed. The method achieves LOQs that are threeâtoâfour orders of magnitude lower than conventional methods while also overcoming matrix interference and maximizing reproducibility.
Experimental
New Methodology Development and Validation: The new method was developed and validated using six azo dyes (para red, dimethyl yellow, sudan I, II, III, and IV) and one non-azo dye (rhodamine B). Five different brands of chili powder spices were obtained from local stores, and standard solutions of each target dye were obtained from SigmaâAldrich, Inc.
Sample Preparation: Each chili powder sample (chili spice 1 through 5) underwent extraction using acetonitrile. The extracts were filtered using 0.22-µm syringe filters and centrifuged to obtain the supernatants for analysis.
Stock and Working Standard: A stock matrix-matched standard was prepared using an aliquot of the chili spice 5 extract spiked to 1250 ppb with the working standard. Chili spice 5 was selected for the matrix blank based on an initial screening that showed no quantifiable amounts of the target analytes. The stock matrix-matched standard was then used for the preparation of a 125âppb matrixâmatched working calibration standard.
Calibration and Matrix Effect Assessment: A 2500 ppb standard was prepared by a fivefold dilution of the 12.5 ppm working standard using 1:1 acetonitrile–water (v/v). A 1-mL aliquot of the 2500 ppb standard was then further diluted to prepare 1250 ppb and 500 ppb standards. 5-µL aliquots of the 500, 1250, and 2500 ppb standards were used to separately spike 500 µL of 1:1 acetonitrile–water (v/v) diluent for final dye concentrations of 5, 12.5, and 25 ppb, respectively. This step was then repeated using the chili spice 5 matrix extract. The spiked modes were compared for any possible matrix effects.
Separation and Quantitation: The sample supernatants and standards were directly injected onto a 3.0 × 100 mm, 2-7âµm Brownlee C8 SPP (PerkinElmer) column for chromatographic separation using a PerkinElmer QSight LX50 UHPLC system, using the Simplicity 3Q software platform for control and quantitation.
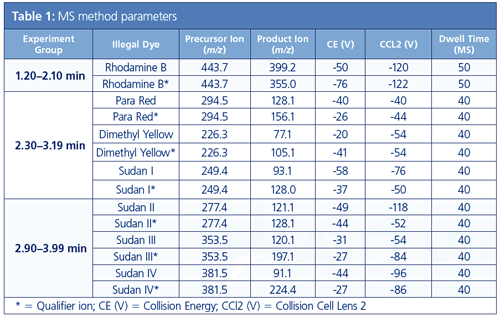
LC Method Parameters: Column: 3.0 × 100 mm, 2-7-µm Brownlee C8 SPP (PerkinElmer); mobile phase: solvent A: 5 mM ammonium formate in water with 0.1% formic acid, solvent B: acetonitrile with 0.1% formic acid; step 1: time: initial, flow rate: 0.8 mL/min, A: 75%, B: 25%; step 2: time: 3.00 min, flow rate: 0.8 mL/min, A: 0%, B: 100%; step 3: time: 4.00 min, flow rate: 0.8 mL/min, A: 0%, B: 100%; step 4: time: 4.50 min, flow rate: 0.8 mL/min, A: 75%, B: 25%; step 5: time: 8.00 min, flow rate: 0.8 mL/min, A: 75%, B: 25%; analysis time: 4 min; re-equilibration time: 4 min; pressure 5000 psi/344.7 bar; oven temperature: 35 °C; injection volume: 3 µL.
MS/MS Source Parameters: Ionization mode: ESI, positive; drying gas: 120; HSID temperature: 320 °C; nebulizer gas: 275; electrospray voltage: 4500 V; source temperature: 420 °C.
Table 1 presents the MS/MS method parameters. All samples were run in triplicate.
Results
The method provided analytical results for the seven target dyes within four minutes with high levels of sensitivity, excellent chromatographic separation, and good recoveries.
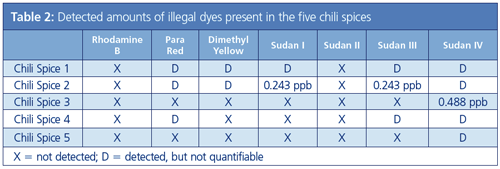
Five of the seven target dyes were detected in one or more chili spices (Table 2), demonstrating the still pervasive presence of these illegal dyes despite their regulatory ban. Sudan IV was detected in all five chili spices. Rhodamine B and sudan II were not detected in any of the chili spices.
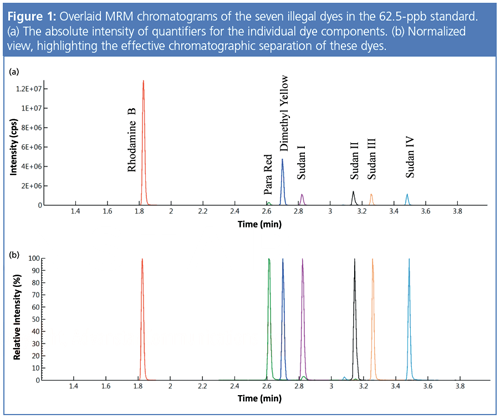
The LOQs were 0.012, 0.092, and 0.972 ppb for rhodamine B, dimethyl yellow, and para red, respectively, and ranged from 0.309 to 0.469 ppb for the four sudan dyes. This is an improvement of three-to-four orders of magnitude over the LOQs provided by conventional methods.
As shown in Figure 1, good chromatographic separation of the seven dyes was achieved in under 4 min, demonstrating the method’s rapid turnaround time and specificity.
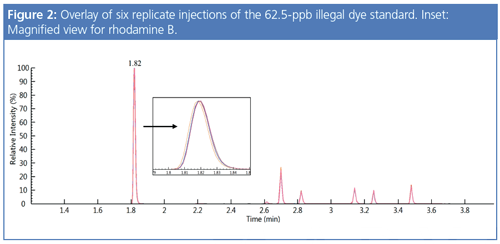
Per Figure 2, the chromatographic overlays of the six replicate injections of the 62.5-ppb dye standard demonstrates the method’s excellent reproducibility.
The overall linear-fit results revealed R2 values in the range of 0.992–0.999, demonstrating very good linearity. Comparing the linear fits with and without matrixâmatched standards, both in slope and overall response, only a marginal matrix effect was evident.
Comparing the recoveries of samples with and without matrix, the experimental QC concentrations were all found to be close to the theoretical values, with recoveries of 92.2–113.6% and CVs of 1.14–15.2%. These results further demonstrate that there is very little matrix effect, particularly ion suppression, for these illegal dyes in chili spice matrix.
Conclusions
This new methodology provides benefits for food producers, distributors, analytical laboratories, and regulators. The much lower LOQs achieved by the new method provides all of these parties with greater confidence in the reported concentration of the illegal dyes in spices. The accuracy and reproducibility provide analytical laboratories with a very reliable method. The absence of matrix effects provides laboratories with additional confidence in their data. The method’s fast turnaround time enables regulators to quickly announce alerts to prevent widespread distribution and sale of contaminated spices.
By using UHPLC combined with MS/MS (aka, triple quad) detection, one is afforded the advantages of both optimum chromatography as well as exceptional analyte sensitivity and selectivity. For applications such as that described herein, this approach is currently unmatched by any other analytical technique.
The ultimate benefits, of course, go to every person who likes spices. Without being aware of it, this new method provides them with safer, healthier food!
References
- T.M. Fonovich, Drug Chem. Toxicol. 36(3), 343–52 (2013).
- M. Stiborova, H.H. Schmeiser, E. Frei, P. Hodek, and V. Martinek, Curr. Drug Metab. 15(8), 829–40 (2014).
- IARC, Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man: Some Aromatic Azo compounds (Lyon, France, Vol. 8, 125–146, 1975).
- S. Tsuda, M. Murakami, N. Matsusaka, K. Kano, K. Taniguchi, and Y.F. Sasaki, Toxicol. Sci.61(1), 92–9 (2001).
- Opinion of the Scientific Panel on Food Additives, Flavorings, Processing Aids and Materials in Contact with Food on a request from the Commission to Review the toxicology of a number of dyes illegally present in food in the EU, Question number EFSA-2005-082. EFSA Journal (2005) 263. www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/263.pdf.
- The World Spice Congress 2012. Sustainability and Food Safety Global Initiatives: Illegal Dyes. www.worldspicecongress.com/uploads/files/28/sess02-c.pdf.
Wilhad M. Reuter joined PerkinElmer 33 years ago, working as a product specialist. For the last 10 years, as a Senior Strategic Applications Specialist, he has been a key contributor in the development and testing of PerkinElmer chromatography systems.

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














