UHPLC Coupled with Fourier Transform Orbitrap for Residue Analysis
LCGC Europe
The trend in residue analysis has changed from target-oriented procedures towards accurate mass full-scan MS techniques. This article describes these developments and addresses the implications of 2002/657/EC.
Food safety is an important issue in the EU and a legal framework covering the entire food chain has been established.1 The central goal of this framework is to guarantee a high level of protection of human health in relation to food. Historically, the analysis of residues in food products is a relatively young discipline. In the Benelux (Belgium, the Netherlands and Luxembourg), residue analysis started in the late 1960s. In most European countries, research on residues and its application in regulatory control of slaughter animals and crop production started even later.
A residue may be defined as a trace of a substance (µg/kg, ng/kg) present in a matrix (e.g., meat, urine, etc.). For any type of animal or food matrix, there are two main groups of substances that must be monitored according to council directive 96/23/EC:2 group A and B substances. Group A comprises the prohibited growth-promoting agents (e.g., hormones, beta-agonists) and the prohibited substances for which no maximum residue limits (MRLs) could be established (Table 1). Group B comprises all registered veterinary drugs, having an MRL, and other residues as summarized in Table 1.

Table 1: Group A and Group B substances.
Control of group A has higher priority than group B because of public-health concern and, therefore, more stringent criteria are implemented. Technical guidelines and performance criteria (e.g., selectivity, specificity) for residue control are described in Commission Decision 2002/657/EC.3 For banned (A) substances the emphasis is on the identification of the substances in a large number of matrices in a concentration as low as possible (zero tolerance principle). In this case, firstly, qualitative methods, targeting multiple residues, need to be developed and, secondly, quantitative methods. For B substances, methods for the quantitative determination of substances in edible matrices need to be developed.
The development of analytical procedures for sample preparation, detection and separation for A and B substances has evolved substantially and these trends will be discussed in this article.
From TLC to UHPLC–MS–MS
In the early 70s thin layer chromatography (TLC) was the method of choice for the qualitative detection of banned substances (thyreostats and certain anabolics at that time). The reasons for this were its specificity, the simplicity of development in two dimensions and the possibility of reaching low limits of detection for an acceptable budget (often using fluorescence detection). The only alternative with acceptable limits of detection — at that time — was gas chromatography with electron capture detection (GC–ECD). High performance liquid chromatography (HPLC) with ultraviolet (UV) detection was introduced in the mid-70s, but the first instruments were expensive and not robust. Also, UV detection did not match the specificity and limits of detection needed for A substances. Fluorescence detectors were only introduced later. However, for the quantitative determination of B substances, UV detection and post-column derivatization was often used. During the 90s, more and more affordable gas chromatography–mass spectrometry (GC–MS) apparatus appeared on the market and the transition from TLC (and HPLC) to GC–MS methods was ongoing. At the end of the 90s, liquid chromatography–mass spectrometry (LC–MS) and LC–MSn belonged more and more to the mandatory standard equipment of a residue laboratory (Figure 1).

Figure 1: Evolution of methods used in residue analysis (adapted from De Brabander et al.6)
From recent review articles,4–6 it may be concluded that LC–triple quadrupole tandem MS (QqQ-MS–MS) is currently the preferred method for residue analysis. The majority of current LC–MS-based hormone and veterinary drug residue analyses relies on the high sensitivity and selectivity of the selected reaction-monitoring (SRM) mode of QqQ-MS–MS. The two-stage mass selection provides the selectivity and sensitivity to enable the detection, identification and quantification of preselected targets at the low µg/kg level in complex biological matrices such as urine, faeces, tissue, feed and hair.4,5 As the cost-effectiveness of analytical procedures is becoming an important parameter for laboratories involved in residue analysis, automation has been introduced to speed up many analytical procedures. Instrumentation to provide for automation is, however, expensive and a distinct number of samples would be required to compensate for the significant capital expenses.
An alternative way to improve cost-effectiveness is to maximize the number of analytes that may be determined by a single procedure. The recent introduction of ultra-high performance liquid chromatography (UHPLC) and fast-switching QqQ-MS–MS instruments significantly increased the number of analytes that can be detected in one run. Indeed, modern instruments produce high signal-to-noise (S/N) ratios, even when relying on short SRM dwell times. This permits monitoring of an increasing number of transitions. For a number of growth promoters (anabolic steroids), the SRM approach is depicted in Figure 2.

Figure 2: UHPLCâQqQ-MSâMS chromatogram obtained from a standard solution of ten anabolic steroids; 0.5 ng injected amount of each analyte. SRM transitions monitored for each component are presented to the right.
Increasing the number of analytes to be monitored further, requires monitoring at specific retention-time windows, also called "timed SRM" or "scheduled SRM". In each retention-time window, the number of transitions to be acquired can be kept relatively low (less than 10). Using this approach, selective and sensitive analysis of a larger number of compounds (maximum 100–120) may be achieved within one single LC–QqQ-MS method. This approach, however, requires frequent readjustment as a result of small shifts in retention time. Another major inherent limitation of targeted LC–MS–MS approaches is the inability to detect residues such as novel illegally administered anabolic steroids designed to escape from control agencies.
Therefore, an attractive alternative is the use of full-scan MS approaches [e.g., time-of-flight (TOF), Fourier transform ion cyclotron resonance (FT-ICR) or Fourier transform Orbitrap]. Full-scan MS approaches offer the possibility to simultaneously analyse a virtually unlimited number of compounds. Furthermore, the retrospective "post-targeted" evaluation of old data offers the possibility to detect non-a priori selected analytes (i.e., no analyte-specific transitions have to be defined before injecting the sample). Moreover, their accurate-mass capabilities support the reconstruction of highly selective, accurate-mass chromatograms of target residues in complex matrices. To allow the detection of residues at the low ppb (mg/kg) or ppt (ng/kg) concentration ranges demanded by legislation, very sensitive full-scan analysers are required.
The medium resolution of TOF systems significantly effects the selectivity and, therefore, the sensitivity gain, compared with unit-resolution scanning MS.5 The utility of high performance TOF mass spectrometric applications has been demonstrated for the multi-compound screening of veterinary drugs in different matrices from animal origin,7,8 but also for doping agents in human urine9 and pesticide residues in crops.10 It should, however, be noticed that accurate-mass determination without proper mass-resolution criteria might lead to false compliant (false negative) results, both in MS screening and MS–MS confirmation.
A lack in mass-resolving power was demonstrated for the anabolic steroid stanozolol analysed using a LC–QTOF-MS.11 Indeed the currently available TOF instruments have mass accuracies of less than 5 ppm and resolutions of maximally 15000 FWHM (full width at half maximum).11 This resolution may lead to inaccurate mass measurements in complex matrices because of unresolved background matrix interferences. Co-elution of isobaric compounds may cause significant deviations in exact mass measurements. This mass resolution is of crucial importance to successfully identify residues or contaminants in samples containing high amounts of complex matrix co-extracts.11 In this context, the high resolving power (up to 100000 FWHM) of the Fourier transform Orbitrap mass spectrometric technology provides precise mass accuracy (below 2 ppm), resulting in both high selectivity and sensitivity for complex sample analysis. This is demonstrated in Figure 3 for the anabolic steroid trenbolone. A minimum resolution of 30000 was required to separate the trenbolone ion from the interfering matrix ion; while complete separation could only be obtained at a resolution of 100000.

Figure 3: Full-scan Orbitrap mass spectra acquired at different resolving powers. Separation of the [M + H]+ ion of trenbolone (m/z = 271.16940) from the ion of a matrix compound (m/z = 271.18811). No separation between analyte ions and matrix compound ions takes place at a resolving power of 10 000.
Van der Heeft et al. observed a similar trend for the determination of steroid esters in hair.12 In their study, the mass-resolving power of 10000 provided by a UHPLC–TOF-MS was too low to resolve analyte ions and co-eluting isobaric compounds from the sample matrix, generating mass measurement results frequently differing more than 5 ppm from the expected values.11
In contrast, the use of a high resolving power (60000) in the UHPLC–Orbitrap MS allows the removal of matrix interferences from isobaric matrix constituents and produces less than 3 ppm mass errors for all steroid esters at all concentration levels.11 Using high-resolving power mass spectrometers, such as the Orbitrap MS, may further improve the confidence in screening results obtained by full-scan accurate mass LC–MS. Figure 4 depicts the reconstructed ion chromatograms of a selection of hormones with very narrow mass tolerance window (< 5 ppm) at 100000 resolving power.

Figure 4: Full-scan Orbitrap reconstructed ion chromatograms of seven selected hormones at resolving power 100 000; 0.5 ng injected amount of each analyte.
Sample Preparation and Separation
Next to improving the detection capability of the instrumentation, the clean-up of the samples before instrumental analysis has also evolved through time. While in the 70s only solvent extraction [liquid–liquid extraction (LLE)] and homemade columns (e.g., silica gel, Al2O3) were used for clean-up, solid-phase extraction (SPE) in the 1980s, immunoaffinity chromatography (IAC) in the 90s and molecular imprinted polymers (MIPs) at the end of the 90s, took over the job.6
Today, more than 80% of all published methods to determine veterinary drug residues apply LLE and/or SPE. Specific combinations of LLE and SPE may be very selective for a certain group of veterinary residues. Selective sample pre-treatment and analysis are, however, very time-consuming and demand a great deal of effort. To maintain sample throughput and cost-effectiveness, it will be necessary in the future to develop generic liquid chromatography–mass spectrometry (LC–MS) screening methods for the simultaneous detection and identification of a wide range of banned and novel hormones, growth promoters, registered veterinary drugs and their metabolites. Due to the broad variability in physicochemical properties of the compounds of interest, the application of a simple clean-up or extraction procedure will be critical to maintain the recovery of the broad range of analytes.
The emerging trend towards accurate-mass and full-scan alternatives, allowing the reconstruction of highly selective accurate-mass chromatograms of target residues in complex matrices, supports this generic sample preparation approach because increased resolving powers allow the discrimination between analytes and matrix interferences. Kaufman et al. described a procedure for the analysis of 100 veterinary drugs in urine and their sample-preparation existed of diluting the urine prior to UHPLC–TOF-MS.13 This approach is, however, not applicable when very low levels of residues need to be determined or when semi-solid or solid samples need to be analysed. Mol et al. developed and validated a generic procedure for the simultaneous extraction of 136 pesticides, 36 natural toxins and 86 veterinary drugs in feed and honey.14 The extraction procedure basically involved an extraction using acidified organic solvent. It may be expected that future initiatives in this line and their application in routine monitoring programmes will drastically reduce both effort and time in residue analysis.
In the multi-residue approach using full-scan MS techniques, the LC part of the system may, however, cause certain limitations. As a result of the large number of different analytes to be separated, run times may become relatively long. This can be overcome by using shorter columns or faster gradients, which on its turn may result in a lower chromatographic resolution. The introduction of pressure-stable 1.7 µm particulate packing materials and novel low-dead-volume, ultra high-pressure (until 105 kPa) LC equipment (UHPLC) provides strategies to improve resolution while maintaining or even shortening run times.
The higher resolution provided by UHPLC may compensate for the selectivity of currently available full-scan accurate mass instrumentation, which is still less than that provided by monitoring MS→MS transitions. One possible disadvantage of using UHPLC is, however, the limited number of spectra, which may be acquired over the peak at a high resolving power (100000) required for discrimination between isobaric interferences and ions of interest. UHPLC peak widths are often only a few seconds long and the scan cycle time at high resolving powers counts more than 1 s per scan.12 Therefore, for most applications resolving powers need to be traded-off against sensitivity.
EU criteria
Future trends in residue analysis will encompass an expansion of the application of UHPLC coupled with accurate mass, high-resolution mass spectrometry for the screening of target residues and for accurate mass confirmation of known and identification of unknown residues and metabolites. However, criteria are absent for these techniques.
According to the 2002/657/EC3,15 MS methods may be used only as confirmatory methods after chromatographic separation (off- or on-line). For LC–MS the 2002/657/EC decision states that suitable LC columns should be used. However, there is no appropriate definition of what "suitable" is. Nowadays, UHPLC columns may offer a comparable separation power to that of capillary columns. Therefore, the time has come to consider the mandatory use of these columns for confirmation. Also, the tolerance of the correspondence of the relative retention time of the analyte to that of the calibration solution [now ±2.5% for (classical) HPLC] should be revised based on existing knowledge on the reproducibility of the retention times in UHPLC.
The 2002/657/EC defines high resolution mass spectrometry (HRMS) as MS at a mass resolution of 10000 according to the 10% valley definition.3 This resolution of 10000 according to the 10% valley rule corresponds to a resolution of 20000 FWHM for modern instruments (e.g. TOF-MS, FT-ICR–MS and FT Orbitrap-MS). The 2002/657/EC has also introduced identification points (IPs) and laid down permitted tolerances for the relative intensities of the detected ions. For the confirmation of Group A and Group B substances a minimum of 4 and 3 IPs are required respectively. The criteria for HRMS are as follows: 2.0 IPs are earned for each precursor ion and 2.5 IPs for each product ion. No criteria for mass accuracy are described. Several studies have, however, demonstrated that false compliant (false negative) results can be obtained when the mass resolving power of the MS is insufficient to separate analyte ions from isobaric co-eluting sample matrix ions.11,12 Based on these data, Nielen et al. proposed additional LC–MS criteria to be implemented in 2002/657/EC (Table 2).3
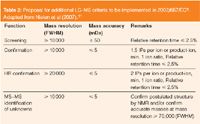
Table 2: Proposal for additional LCâMS criteria to be implemented in 2002/657/EC3. Adapted from Nielen et al (2007).11
This proposal acknowledges the performance of state-of-the-art LC–TOF-MS instruments by ascribing 1.5 IPs per ion versus 1.0 in conventional low-resolution LC–MS,3 but does not allow the use of only one parent and product ion, which should remain the exclusive domain of HRMS.11 For the identification of metabolites or unknown compounds a higher resolution is warranted when NMR cannot be obtained. According to Nielen et al.11 the proposed resolution of ≥70000 (FWHM), will assure that reliable elemental compositions of product ions differing in one CO, C2H4 or N2 substructure can be obtained until m/z = 400. In some cases a resolution of 100000 may even be necessary to achieve discrimination of product ions from matrix interferences, in particular when using more generic sample preparation procedures and multi-residue procedures.
Conclusions
There has been an increased interest in methods that simultaneously analyse various classes of veterinary drugs, hormones etc. In one run, such multi-residue analytical procedures may screen for more than 100 compounds, but full-scan accurate mass instruments (e.g., TOF-MS, FT Orbitrap-MS) are required to do this. LC–QqQ-MS (using selected SRM transitions) is still the most frequently applied technique in residue analysis. But today, in theory, all components can be measured by full-scan MS, not requiring previous acquisition of ion transitions and allowing post-acquisition interpretation of data. In the future, the most important part of the method development will, therefore, shift from detection optimization to the development of generic extraction of the compounds of interest from the matrix.
This will undoubtedly lead to increased innovations in sample preparation techniques. Finally, the introduction of new MS techniques also has consequences for the EU criteria defined for confirmation of compound identity. Identity confirmation is influenced by both mass accuracy and mass resolution. Therefore, a discussion should be initiated, ultimately leading to appropriate criteria for accurate mass techniques and revision of Commission Decision 2002/657/EC.
Lynn Vanhaecke has a PhD in bioscience engineering from the Ghent University and is currently appointed as doctor-assistant at the Department of Veterinary Public health and Food Safety. The chemical analyses of food and the analysis of residues and contaminants in biological matrices relating to human health are her core research objectives.
Karolien Verheyden is a last-year PhD student whose research focuses on the analysis and metabolism of phytosterols to elucidate the occurrence of boldenone and related steroids in cattle.
Julie Vanden Bussche is a PhD student whose research deals with the optimization of new analytical approaches for the discrimination of exogenously administered and endogenously formed thyreostats in livestock.
Frans Schoutsen is European product specialist at Thermo Fisher Scientific.
Hubert De Brabander is professor at the Ghent University and dean of the Faculty of Veterinary Medicine. He has over 35 years experience in residue analysis in animal and food matrices.
References
1. White Paper on Food Safety, Commission of the European Communities, Brussels, http://ec.europa.eu/dgs/health_consumer/library/pub/pub06_en.pdf (2000).
2. Council Directive 1996/23/EC, Off. J. Eur. Commun., L125, 10 (1996).
3. Commission Decision 2002/657/EC, Off. J. Eur. Commun., L221, 8 (2002).
4. H.F. De Brabander et al., J. Mass Spectrom., 42(8), 983–998 (2007).
5. A.A.M. Stolker, T. Zuidema and M.W.F. Nielen, Trac-Trends Anal. Chem., 26(10), 967–979 (2007).
6. H.F. De Brabander et al., J. Chromatogr. A, in press (2009).
7. A. Kaufmann et al., J. Chromatogr. A, 1194(1), 66–79 (2008).
8. A.A.M. Stolker et al., Anal. Bioanal. Chem., 391(6), 2309–2322 (2008).
9. M. Kolmonen et al., Anal. Chim. Acta., 585(1), 94–102 (2007).
10. A.R. Fernandez-Alba and J.F. Garcia-Reyes, Trac-Trends Anal. Chem., 27(11), 973–990 (2008).
11. M.W.F. Nielen et al., Anal. Chim. Acta., 586(1–2), 122–129 (2007).
12. E. van der Heeft et al., J. Am. Soc. Mass Spectrom., 20(3), 451–463 (2009).
13. A. Kaufmann et al., Anal. Chim. Acta., 586(1–2), 13–21 (2007).
14. H.G.J. Mol et al., Anal. Chem., 80(24), 9450–9459 (2008).
15. F. André et al., Trac-Trends Anal. Chem., 20(8), 435–445 (2001).
Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.











