TSKgel G3000SWXL Columns for the Reproducible Analysis of Monoclonal Antibodies and Proteins
Tosoh Bioscience LLC
Atis Chakrabarti, PhD, Tosoh Bioscience LLC
Gel filtration chromatography (GFC) is popular among biochemists for the isolation of proteins and monoclonal antibodies. With 5 μm particles and 250 Å pores, TSKgel G3000SWXL columns are an excellent choice for small protein and peptide separations. TSKgel SWXL columns feature rigid spherical silica particles, the surface of which has been shielded from interacting with proteins by chemical derivatization with ligands containing proprietary diol functional groups. Tosoh's proprietary surface chemistry provides an inertness, which allows for minimal adsorption of proteins and other protein aggregates.
TSKgel G3000SWXL columns also feature high pore volume per unit column volume, low sample adsorption, excellent column efficiency, and very well-defined pore size distribution. All of these factors contribute to unsurpassed sample resolution. This application note reports the preliminary analysis of monoclonal antibodies (mAb) using a TSKgel G3000SWXL, 5 μm, 7.8 mm ID × 30 cm column, demonstrating the effectiveness of the proprietary surface chemistry.
Experimental Conditions
Analyses were carried out using an Agilent 1200 HPLC system run by Chemstation (ver B.04.02).
Column: TSKgel G3000SWXL, 5 μm, 7.8 mm ID × 30 cm; lot T03294-19S
Mobile phase: 100 mmol/L KH2PO4/Na2HPO4, pH 6.8,
100 mmol/L Na2SO4 + 0.05% NaN3
Flow rate: 1.0 mL/min
Detection: UV @ 280 nm
Temperature: ambient
Injection vol.: 20 μL
Sample: monoclonal antibody (BI-mAb-02)
High purity HPLC grade Sigma Aldrich chemicals were used in this study. All the standards and samples were filtered through a 0.45 μm membrane before injecting into the column.
Results and Discussion
Figure 1 shows the overlay of the first two consecutive injections of a monoclonal antibody (BI-mAb-02) onto a TSKgel G3000SWXL column. This brand new column was not pre-conditioned prior to this analysis. The monomer peak is baseline resolved from the dimer peak and the aggregate peaks. The overlay does not show any difference in the monomer and dimer peak areas of the first and second injections. There is no shift in retention time between the two consecutive injections and no difference in peak height is noticeable. These results clearly demonstrate that there was not any adsorption of the monoclonal antibody onto the column. Table I summarizes the results of the analysis of a monoclonal antibody used in this study.
Figure 1: Analysis of BI-mAb-02 using a TSKgel G3000SWXL column â an overlay of the first two injections.
Similar results as those detailed above were obtained with another TSKgel G3000SWXL column from a different lot (T03061-18S). The number of theoretical plates in the analysis of BI-mAb-02 was 4125 and 4100 for the monomer peak for column lots 18S and 19S respectively. Both of the columns were also tested using a standard protein mixture containing thyroglobulin (0.5 g/L), γ-globulin (1 g/L), ovalbumin (1 g/L), ribonuclease A (1.5 g/L), and PABA (0.01 g/L) and compared with the recommended values mentioned in the operating conditions and specifications (OCS) sheet supplied with the column (data not shown here). The efficiency, as measured by the number of theoretical plates for PABA (para-amino benzoic acid), was >32,000 for the columns from both lots. This is well above the quality control specification value of >20,000. The asymmetry factor was 1.18, which is well within the quality control specification range of 0.7–1.6. Repeated injections produced excellent reproducibility. Preliminary results with BI-mAb-01, human IgG, and mouse monoclonal IgG also did not show any difference between two consecutive injections using the TSKgel G3000SWXL columns from lots 18S and 19S (data to be published elsewhere).
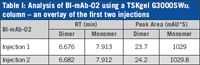
Table I: Analysis of BI-mAb-02 using a TSKgel G3000SWXL column â an overlay of the first two injections
Conclusions
As demonstrated in this application note, two consecutive injections on brand new, non-preconditioned TSKgel G3000SWXL columns did not show any difference in monomer and dimer peak areas, retention times, and peak heights with the analysis of a monoclonal antibody. The TSKgel G3000SWXL columns did not show any adsorption of the monoclonal antibody, leading to accurate quantitation of the mAb content.
Tosoh Bioscience and TSKgel are registered trademarks of Tosoh Corporation.
Tosoh Bioscience LLC
3604 Horizon Drive, Suite 100, King of Prussia, PA 19406
tel. (484) 805-1219, fax (610) 272-3028
Website: www.tosohbioscience.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.










