Troubleshooting for Two-Dimensional Liquid Chromatography: Breakthrough in the Second Dimension
A common problem encountered in the development of 2D-LC methods is that the first dimension mobile phase properties can negatively affect the quality of subsequent second dimension separations. This installment reviews the origin of this problem and discusses potential solutions.
Two-dimensional liquid chromatography (2D-LC) separations can pose troubleshooting challenges not normally encountered in conventional high-performance liquid chromatography (HPLC) separations. A common problem encountered in the development of 2D-LC methods is that the first dimension mobile phase properties can negatively affect the quality of subsequent second dimension separations. In this instalment the origin of this problem and potential solutions are discussed.
For more than three decades, this “LC Troubleshooting” column has focused on solving problems encountered in liquid chromatography methods involving a single column. Since the late 1970s, researchers have been working to develop two-dimensional liquid chromatography (2D-LC), which typically involves two different columns (1). For decades, 2D-LC has been used extensively and successfully in several specific application areas, including proteomics and polymer separations. Most recently, however, the number of commercially available options for off‑the‑shelf instrumentation for 2D-LC has increased. Because of this, users in a broad array of application areas have begun deploying 2D separations more widely (2). Given the steady increase of active users deploying 2D-LC, it is now time to dedicate some instalments of “LC Troubleshooting” to problems encountered in 2D-LC. Although it is true that many of the troubleshooting topics in the context of conventional HPLC separations also apply to 2D‑LC (for example, best practices for mobile‑phase preparation are nominally the same for 1D- and 2D-LC), it is also true that there are challenges that are unique to 2D-LC and deserve focused attention. Readers interested in learning much more about state‑of‑the‑art 2D-LC separations are referred to several recent review articles (1–3). In the following paragraphs, I provide a brief review of the concept of 2D separation, and then discuss one of the common problems encountered by users developing 2D-LC methods: breakthrough peaks in the second dimension.
Two-Dimensional Separations: The Basic Idea
People are interested in 2D-LC separations because the 2D format provides a chance to resolve analytes that otherwise would not be resolved by a single column in conventional 1D‑LC. This can be useful for separating very complex mixtures, such as tryptic digests of proteins where the analyst tries to extract as much information from the sample as possible. However, 2D separations are also useful for samples that are not so inherently complex, but contain several analytes that are difficult to separate. For example, samples of several different enantiomer pairs may be difficult to separate using a single chiral column, but may be relatively easy to separate in a 2D format using a combination of achiral and chiral columns (4).
There are several different modes or ways of executing 2D‑LC separations. Among these, the single heartcut mode—denoted LC‑LC—is the simplest in terms of the instrumentation, methods, and data analysis involved. The goal of such a separation is illustrated in Figure 1. A first dimension (1D) separation results in three clusters of unresolved peaks. The first cluster contains a peak corresponding to the analyte of interest (blue peak). Current commercial instrumentation and software make it straightforward to target this peak and instruct the instrument to transfer a portion of effluent from the 1D column containing the peak (along with the overlapping pink and green peaks) to a second dimension (2D) column for further separation. Then, provided a 2D column and separation conditions are chosen that are complementary to the 1D conditions, the analyte of interest can be separated from the compounds it overlapped with in the 1D separation, enabling accurate quantitation and a qualitative analysis free from interferents.

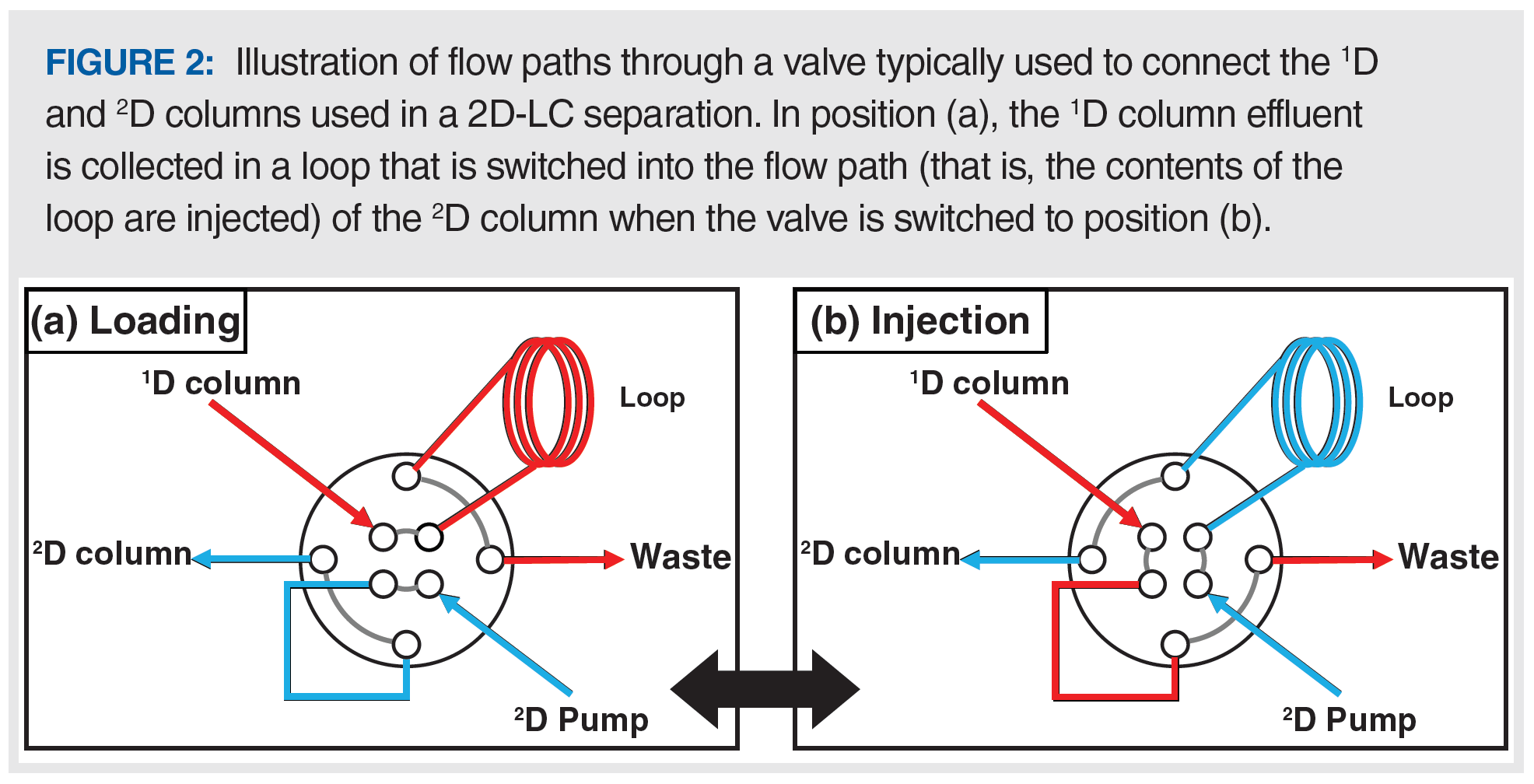
There are a number ways that instrumentation can be configured to support such a separation, but one example of the interface between the 1D and 2D separations is shown in Figure 2. The interface valve has two positions, and the valve is toggled to either have the 1D column effluent flow to waste in regions of the separation that don’t require additional 2D separation (position B), or to collect the 1D effluent in the sample loop (position A). At the end of this sampling period, the contents of the loop are effectively injected into the 2D column for further separation by switching the valve from position A to B.
Mobile-Phase Mismatch Can Lead to Breakthrough Peaks
As indicated in the preceding section, an important determinant of successful 2D-LC separations is the degree of complementarity of separation conditions (that is, the separation selectivities should be different) used in the first and second dimensions. Sometimes, this can be realized without the conditions used in the 1D separation negatively impacting the quality of the 2D separation as measured by chromatographic efficiency or detection sensitivity. For example, many applications of 2D‑LC in the field of proteomics involve a 1D separation based on a cation-exchange mechanism and a 2D separation that relies on a reversed‑phase mechanism. In the case of 2D-LC, peptides are eluted from the 1D column in effluent composed mainly of a buffered aqueous solution. This is highly favourable for the 2D separation in the sense that relatively large volumes of this effluent can be injected into the 2D column without negatively impacting the widths or shapes of 2D peaks. However, a favourable relationship between the operating conditions used in the two dimensions is not inevitable. In other cases, the properties of the effluent from the 1D column can have a dramatic and negative effect on the quality of the 2D separation. We refer to problems of this type as originating from mismatch between the mobile phases used in the two dimensions. An example of this is where we use a hydrophilic-interaction liquid chromatography (HILIC) separation in the first dimension and a reversed‑phase liquid chromatography (RPLC) separation in the second dimension. In general, HILIC separations tend to involve mobile phases contaning much more than 50% acetonitrile, whereas RPLC separations of compounds that can be reasonably retained under HILIC conditions tend to involve mobile phases containing much less than 50% acetonitrile. If HILIC separation is used in the first dimension and RPLC separation is used in the second dimension, this then sets up a situation where a large volume of 1D effluent containing analytes of interest and more than 50% acetonitrile is injected into the RPLC column running with a mobile phase containing less than 50% acetonitrile.
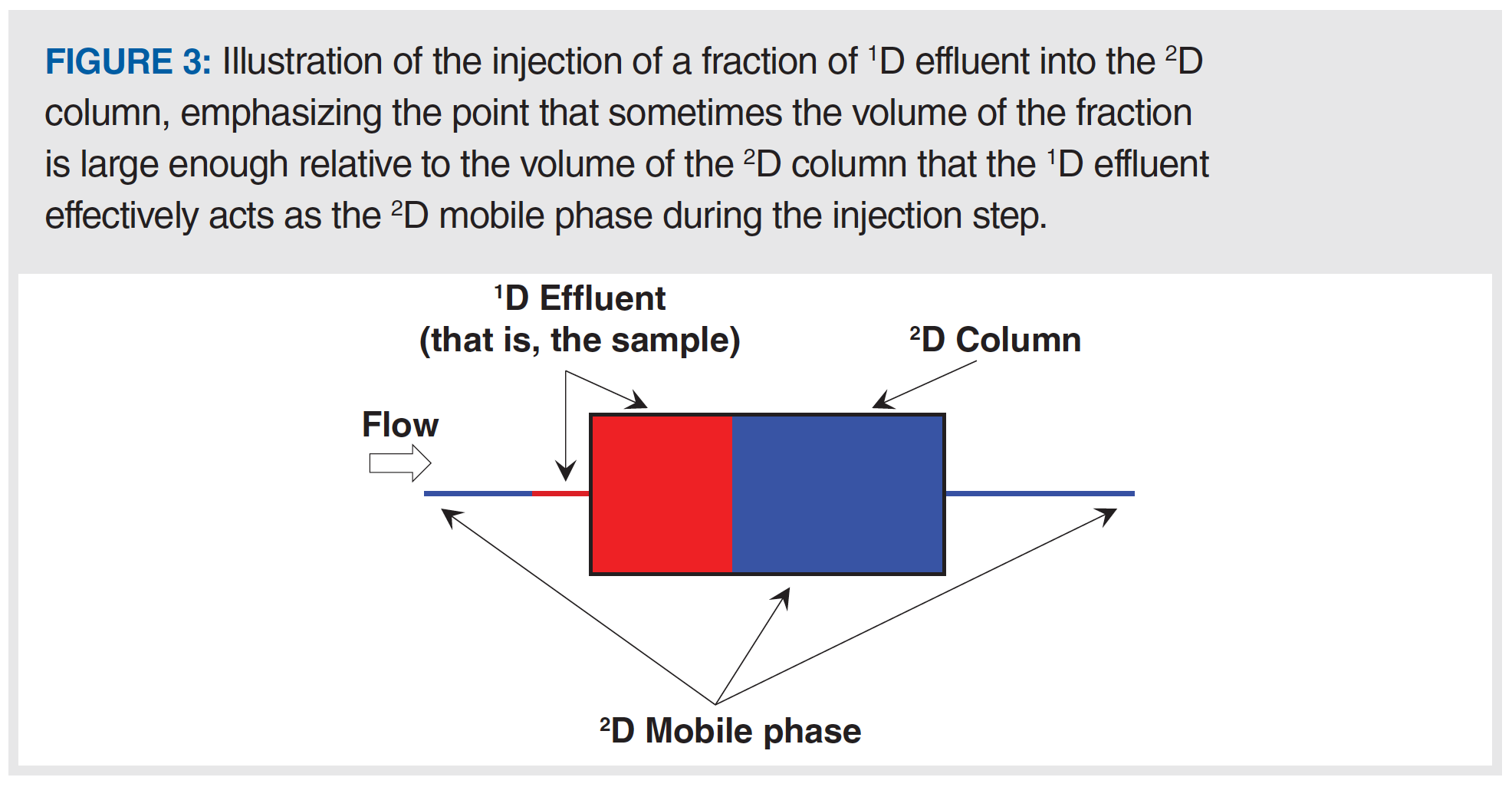
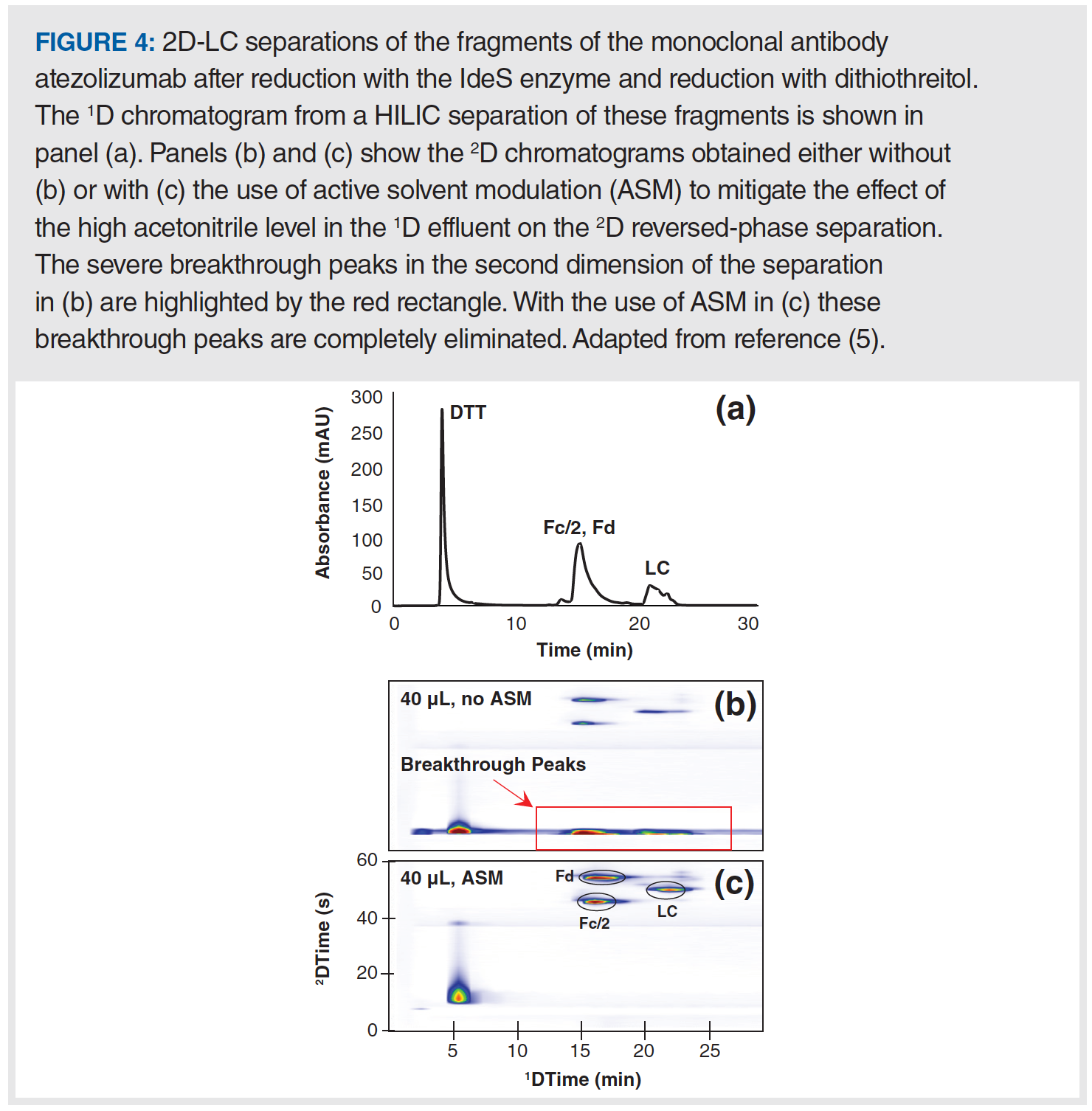
Figure 3 shows an illustration of the local environment inside the 2D column during the injection of 1D effluent into the 2D column. In principle, this injection step is not different from what happens in conventional 1D-LC. However, problems can arise in 2D-LC when the volume of effluent from the 1D column is not very small (less than 1%) relative to the volume of the 2D column itself (which is common in 2D-LC). I like to emphasize the impact of this difference by saying that in 2D-LC the 1D column effluent becomes the 2D mobile phase during the injection step. If analytes are weakly retained by the 2D column in the 1D effluent, then it hardly matters what the analyte retention is in the 2D column in the 2D mobile phase—retention during the injection step will be determined primarily by the properties of the 1D effluent. Therefore, such conditions can very easily lead to terrible chromatographic outcomes in the second dimension. Figure 4 shows the experimental results from recent work by my research group that was aimed at developing 2D-LC separations of monoclonal antibodies (mAbs) using HILIC and RPLC separations in the first and second dimensions, respectively. In this instance, the influence of the 1D effluent on the 2D separation is so severe that most of the analyte mass injected into the 2D column breaks through in the dead volume of the column, and only a small fraction of the mass is retained and eluted with normal looking peaks. This type of outcome is, of course, highly undesirable from the point of view of both quantitative and qualitative uses of 2D-LC.
A Variety of Solutions to Help
While the mobile-phase mismatch problem described above is certainly not new to the 2D-LC community, its significance in practical 2D-LC method development and the importance of developing solutions for the problem to the continued growth of the technique have garnered a lot of attention in recent years. A broad perspective on the significance of the problem in different application areas and for possible combinations of different separations modes has been discussed in detail by Pirok and co-workers (6). The good news is that currently there are commercially available technologies that can be used to address the problem, and other potential solutions are being explored by various research groups across the globe (7). Currently, two of the most effective commercially available solutions rely on interventions that disrupt the situation illustrated in Figure 3 that can lead to poor outcomes like that shown in Figure 4b, by adjusting the composition of the 1D effluent prior to injection of that material into the 2D column for further separation. In an approach known as at-column dilution (ACD), a pump is used to add diluent to the 1D column effluent as it exits the column, thereby adjusting the properties of the effluent prior to loading into the 2D column (8). In a different approach known as active solvent modulation (ASM), valve technology is used to enable temporary adjustment of the properties of the 1D effluent prior to injection into the 2D column (9). Figure 4 shows a representative result from our own work aimed at demonstrating the positive impact of an intervention like this on the quality of the 2D separation in a situation where the impact of the mobile-phase mismatch is severe if left unchecked. A 1D HILIC separation of the fragments of the monoclonal antibody atezolizumab after treatment with the IdeS enzyme and reduction with dithiothreitol is shown in panel (a). Adding a 2D RPLC separation either without or with ASM produces the 2D chromatograms shown in panels (b) and (c), respectively. Separating these protein fragments by HILIC requires a 1D mobile phase with about 70% acetonitrile, and a 2D mobile phase with about 30% acetonitrile for RPLC separation. When there is no mitigation of the effects of this mobile-phase mismatch, the 70% acetonitrile in the 1D effluent causes severe breakthrough of protein peaks in the dead volume of the 2D column due to very low RPLC retention in a high acetonitrile environment. These breakthrough peaks are highlighted by the red rectangle in Figure 4b. On the other hand, ASM enables a reduction in the acetonitrile level to below 30% in the sample that is injected into the 2D column, thereby completely eliminating the breakthrough, as shown in Figure 4c.
Summary
Currently, it is most important that 2D‑LC users recognize that breakthrough in the second dimension of 2D-LC separations can be a severe problem, particularly in cases where the degree of mismatch between the mobile phases used in the two dimensions is large. Currently, ACD and ASM are two popular and commercially available solutions that address many, but not all, of these challenges. There are many other potential “homemade” solutions, and several research groups are exploring other potential solutions involving membrane and sorbent-based approaches, to name a few (7,10). Given the importance of this problem it seems likely that we will see other commercial solutions emerge in the not-too-distant future.
References
- D.R. Stoll and P.W. Carr, Anal. Chem. 89, 519–531 (2017). https://doi.org/10.1021/acs.analchem.6b03506.
- B.W.J. Pirok, D. Stoll, and P.J. Schoenmakers, Anal. Chem. 91, 240–263 (2019). https://doi.org/10.1021/acs.analchem.8b04841.
- D.R. Stoll, K. Zhang, G.O. Staples, and A. Beck, Adv. Chromatogr. 56, 29–63 (2018).
- C.J. Venkatramani, L. Wigman, K. Mistry, and N. Chetwyn, J. Sep. Sci. 35, 1748–1754 (2012). https://doi.org/10.1002/jssc.201200005.
- D.R. Stoll, D.C. Harmes, G.O. Staples, O.G. Potter, C.T. Dammann, D. Guillarme, and A. Beck, Anal. Chem. 90, 5923–5929 (2018). https://doi.org/10.1021/acs.analchem.8b00776.
- B.W.J. Pirok, A.F.G. Gargano, and P.J. Schoenmakers, J.Sep. Sci. 41, 68–98 (2017). https://doi.org/10.1002/jssc.201700863.
- D. Stoll, in Handbook of Advanced Chromatography/Mass Spectrometry Techniques, M. Holcapek and Wm.C. Byrdwell, Eds. (Elsevier, London, 2017), pp. 227–286.
- B. Koshel, R. Birdsall, and W. Chen, J. Chromatogr. B. 1137, 121–906 (2020). https://doi.org/10.1016/j.jchromb.2019.121906.
- D.R. Stoll, K. Shoykhet, P. Petersson, and S. Buckenmaier, Anal. Chem. 89, 9260–9267 (2017). https://doi.org/10.1021/acs.analchem.7b02046.
- Y. Chen, L. Montero, and O.J. Schmitz, Anal. Chem. 120, 115–647 (2019). https://doi.org/10.1016/j.trac.2019.115647.
Dwight R. Stoll is the editor of “LC Troubleshooting.” Stoll is a professor and the co-chair of chemistry at Gustavus Adolphus College in St. Peter, Minnesota, USA. His primary research focus is on the development of 2D-LC for both targeted and untargeted analyses. He has authored or coauthored more than 70 peer-reviewed publications and four book chapters in separation science and more than 100 conference presentations. He is also a member of LCGC’s editorial advisory board. Direct correspondence to: amatheson@mjhlifesciences.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)