Transfer of USP Tolazamide Normal Phase HPLC Method to SF Using the Agilent 1260 Infinity Hybrid SFC/UHPLC System
Agilent Technologies
Normal-phase liquid chromatography (LC) methods often have long run-times and use environmentally toxic/costly solvents. This application demonstrates the transfer of tolazamide normal phase method to a supercritical fluid chromatography (SFC) method leading to savings in analysis time and solvent consumption.
Introduction
Tolazamide is an oral, blood glucose lowering drug of the sulfonylurea class. The USP assay method for tolazamide uses a normal phase method and includes chloroform as a sample diluent and hexane as the mobile phase. Chloroform is a known carcinogen, potentially toxic to analysts and expensive to dispose of. Hexane is included in the list of chemicals on the US Toxic Release Inventory (TRI). SFC uses less hazardous chemicals such as supercritical carbon dioxide as a major component of the mobile phase and methanol as a sample diluent. Due to low viscosity of the supercritical carbon dioxide, SFC methods offer faster separation without losing efficiency and faster column re-equilibration.
Experimental
The Agilent 1260 Infinity hybrid SFC/UHPLC system was used to perform both the normal phase as well as the SFC method on the single instrument. The normal phase method and the SFC method also used the same column — Agilent ZORBAX Rx-SIL 4.6 × 250 mm, 5 µm column. The SFC method uses methanol as modifier with gradient starting from 0% B to 12% B.
Results
A reduced analysis time was observed when analysing tolazamide using the SFC method (Figure 1), in addition to a reduction in solvent consumption. A 4× decrease in analysis time and a 19× decrease in cost were achieved with the SFC method for every analytical run. Assuming analysis time to be US $ 80/h, the overhead cost would decrease to US $ 20/h.

Figure 1: Separation of standard solution of tolazamide and its internal standard, tolbutamide, using the normal phase and supercritical fluid chromatography (SFC) method.
The system suitability test was performed using both methods. The SFC method provided similar resolution and better precision compared to the normal phase method (Table 1). In addition, SFC chromatographic runs produced sharper and narrower peaks compared to normal phase methods. The tolazamide peak width at half height is 0.04 min, 7× lower compared to the normal phase method (Table 2).

Conclusion
The tolazamide USP normal phase method was transferred to a SFC method using the Agilent 1260 Infinity SFC/UHPLC system. The robust SFC method meets USP system suitability criteria; is 4× faster, 19× less expensive and displays enhanced peak properties compared to the normal phase method.
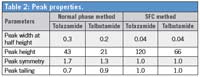
Table 2: Peak properties.
Reference
(1) S.S.Lateef and Vinayak.A.K, Agilent Application Note. 5991-0395EN (2012).
Agilent Technologies Inc.
5301 Stevens Creek Blvd, Santa Clara, California 95051, USA
tel. (800) 227 9770 (Directory) fax (866) 497 1134
Website: www.agilent.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.









