Trace Perchlorate Determination by Ion Chromatography
The Column
Perchlorate salts are relatively stable, soluble in water, and migrate into groundwater sources causing possible challenges for drinking water suppliers as dissolved perchlorate has been identified to impair normal thyroid function. The development of a sensitive analytical method for perchlorate determination is therefore important to protect public health. This article addresses the validation of ion chromatography with suppressed conductivity detection (IC–CD), applying isocratic elution to analyze perchlorate.
Photo Credit: Jose A. Bernat Bacete/Getty Images

Maike A. Seiler1,2, Detlef Jensen3, Udo Neist1, Ursula K. Deister2, and Franz Schmitz1, 1Hessian State Laboratory, Wiesbaden, Germany, 2Hochschule RheinMain, Rüsselsheim, Germany, 3Thermo Fisher Scientific, Dreieich, Germany.
Perchlorate salts are relatively stable, soluble in water, and migrate into groundwater sources causing possible challenges for drinking water suppliers as dissolved perchlorate has been identified to impair normal thyroid function. The development of a sensitive analytical method for perchlorate determination is therefore important to protect public health. This article addresses the validation of ion chromatography with suppressed conductivity detection (IC–CD), applying isocratic elution to analyze perchlorate.
Perchlorate is an environmental contaminant of both anthropogenic and natural origin found in water of varying sources (1). The natural origin of perchlorate was found to be atmospheric deposition when natural events, such as thunderstorms, contaminate groundwater (2). Anthropomorphic origins for perchlorate result from its usage in rockets, missiles, fireworks, pyrotechnics, flares, matches, ordnance, and explosives (3). Once ingested, perchlorate reduces iodine uptake in the thyroid gland, impairing its function and therefore the production of important thyroid hormones (4,5). The derived health disorder, hypothyroidism, is particularly dangerous during pregnancy because it poses risks for the physical and mental development of the unborn child (6).
In 2011, the US Environmental Protection Agency (EPA) determined that perchlorate meets the Safe Drinking Water Act criteria for regulation as a drinking water contaminant because it may adversely affect health if consumed, and concluded that a decrease in perchlorate contamination is a real opportunity for health risk reduction (7). The EPA recommends a provisional advisory drinking-water limit of 15 µg/L of perchlorate (8). Tests undertaken in Germany showed perchlorate levels in groundwater close to intensely farmed areas were up to a value of 6 µg/L, while swimming pool water samples were as high as 980 µg/L (9). By far the highest concentration of perchlorate found was in pore water samples from the Maifeld area in Berlin (Germany) that showed values of up to 15,000 µg/L following firework displays (10). Perchlorate contamination of agricultural soils and products from the use of already polluted surface and groundwater sources for irrigation may be assumed, as well as the contamination of perchlorate in food from after-crop disinfection techniques for fruit and vegetables (11). The determination of perchlorate is therefore of the highest interest for public health.
As a consequence, several organizations, such as the US EPA, have developed methods for the determination of perchlorate in food and drinking water matrices that include techniques such as ion chromatography with conductivity detection (IC–CD), with heart-cutting techniques, or IC coupled to electrospray ionization and mass spectrometry (IC–ESI-MS).
This article focuses on perchlorate determinations that are being performed by direct sample injection without heartâcutting techniques, using standard analytical IC equipment. It refers to extended validation experiments based on the EPA 314.0 (12) method in support of ongoing ISO development work (13). The aim of this study is to address laboratory workflow needs, as well as the user’s analytical and financial requirements. This should support a broader acceptance of a nascent ISO standard method, and facilitate its integration into the method collection of interested laboratories. The method was applied to the determination of perchlorate in arbitrarily selected real-world samples, which included drinking, raw, swimming pool, surface, and waste- waters.
Experimental
Instrumentation and Analysis: The water used fulfilled the requirements of ISO 3696, Grade 1 (14), and the anion stock standard solutions for chloride, sulphate, and perchlorate were of p.a. quality. Perchlorate solution from different sources was used either for the method validation and calibration experiments, or as an independent source for quality control. The chromatographic instrument consisted of a gradient pump with a flow of 0.25 mL/min, an autosampler with 1000 µL injection loop, a column thermostat set at 30 °C, an eluent generator delivering 35 mmol/L KOH, and a conductivity detector (Thermo Fisher Scientific). The column was a Dionex IonPac AS20 with guard column (Thermo Fisher Scientific), and for suppression a Dionex AERS (Thermo Fisher Scientific) was used, all in the 2-mm format. The total run time was 30 min for each sample. Performance and calibration data, as well as the working range, were statistically evaluated for a concentration decade analyzing 10 standard solutions of different perchlorate concentrations using ISO standard methods (15,16). For routine analysis, a minimum of 5 concentration levels were calibrated each working day for the defined range.
Results and Discussion
The initial calibration of the given concentration decade was performed by repetitive injection of the 10 calibration solutions with different injection volumes.
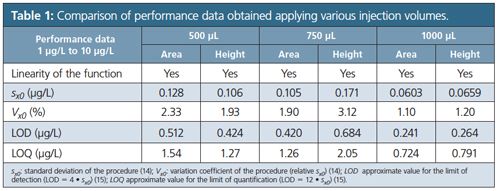
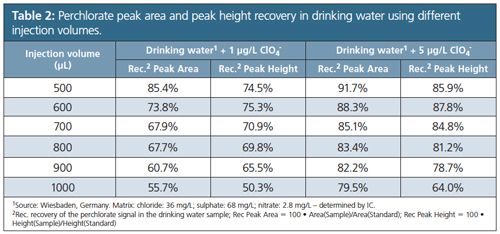
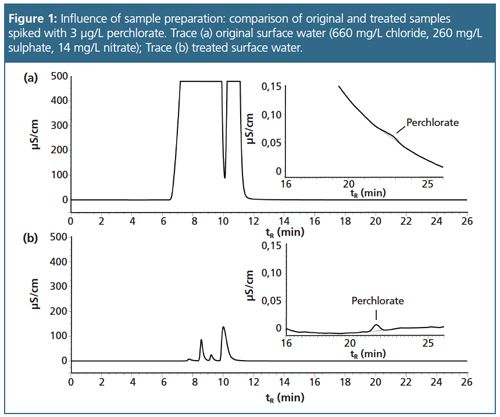
The statistical results for both peak area and peak height are summarized in Table 1. In order to examine possible matrix effects perchlorate-free local drinking water was spiked with 1 µg/L and 5 µg/L perchlorate and repetitively injected with injection volumes between 500 µL and 1000 µL. Peak areas and peak heights were compared to those of diluted and matrixâfree standard solutions of the perchlorate concentrations applying identical conditions, with Table 2 summarizing the evaluation. For both concentration levels peak area recovery and peak height recovery decreased with increasing injection volumes. With increasing matrix ion concentration perchlorate turns into a rider peak on the tailing flank of the matrix sum peak (Figure 1). The overall impact on recovery depends on the analyte concentration, and recovery at lower concentrations is below that at higher concentrations. Based on the data displayed in Tables 1 and 2 the working range was established from 1.5 µg/L to 15 µg/L perchlorate with an injection volume of 750 µL. LOD was 0.33 µg/L, LOQ resulted in 0.98 µg/L, and Vx0 was determined at 1.0%.
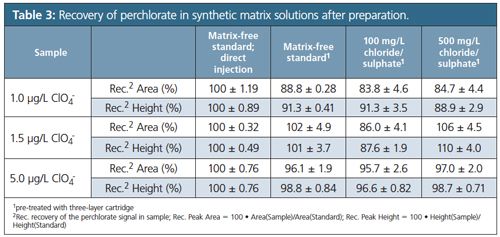
Anions such as chloride, sulphate, and carbonate, as well as metals, can be removed using cation exchanger cartridges in the Ag-, Ba-, and H-form (17). In order to mobilize barium ions off the resin for the precipitation reaction with sulphate, the sample needs to contain competing cations, like calcium. The competing cations can either be present in the sample or be added as a calcium chloride solution. Calcium chloride solution was always added to all samples with unknown calcium loads in our studies. Table 3 summarizes the recovery of perchlorate in synthetic matrix solutions after sample preparation, combining different analyte and matrix ion concentrations. The result obtained for a matrix-free and untreated perchlorate standard solution served as reference for the calculation of the recovery rates. Recovery values were in the range of 84% to 110% for chloride and sulphate, which is within the EPA 314.0 range (12).
Randomly selected real-world samples including drinking, raw, swimming pool, surface, and waste waters of different origins were analyzed accordingly (Table 4). Samples showing results below 1.5 µg/L perchlorate were spiked to ensure a perchlorate concentration above the lower working range. All the swimming pool water samples and one of the surface water samples had to be diluted into the analytical working range. Despite being treated with non-polar RP-C18 cartridges, the chromatograms for the six wastewater samples showed noisy and nonâreproducible drifting baselines or signals in the retention time window of perchlorate, preventing a reliable identification of perchlorate even after standard addition experiments. The interfering components were exclusively observed in clarified wastewater samples of domestic and industrial origin. It is apparent that those unknowns could be organic acids of complex composition. Alternative approaches, such as IC–MS/MS or heart-cutting techniques for instance, should be applied in case of such interferences.
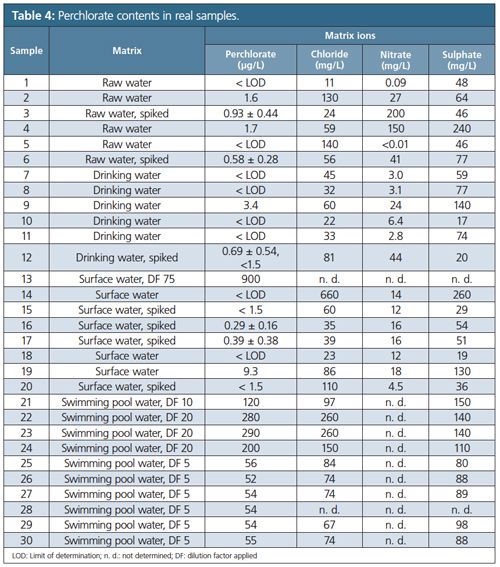
Four raw water samples, two drinking water samples, six surface water samples, and all of the swimming pool samples contained perchlorate. For sample 13 (see Table 4) and all of the swimming pool samples a simple dilution sufficed as sample preparation. Samples 12, 15, and 20 showed signals between the LOQ and LOD. The quantitative results from standard calibration experiments below 1.5 µg/L (samples 3, 6, 16, and 17) are reported including the expanded measurement uncertainty. Sample 17 showed a measurement uncertainty of up to 100% while samples 12, 15, and 20 showed uncertainties over 100%, which is why they are reported as < 1.5 µg/L (18).
Conclusion
This extended method validation shows the applicability of IC–CD for trace perchlorate determination in water samples of different origin, therefore supporting the development of a respective international standard pursued by the ISO. The described procedure is easy to implement, eluent preparation and sample preparation is simplified, resulting in convincing analytical performance with a LOD of 1.5 µg/L for perchlorate and excellent chromatographic performance in terms of resolution and lifetime. The method could serve as an alternative, if more sophisticated IC techniques such as gradient elution, heart-cutting, or coupling techniques
(IC–MS) are not available.
References
- M.A. Seiler et al., Environmental Sci. Eur.28(18), (2016).
- P.K. Dasgupta et al., Environ. Sci. Technol.39, 1569–1575 (2005).
- C.W. Trumpolt et al., Rem J. 16, 65–89 (2005).
- J.B. Stanbury and J.B. Wyngaarden, Metabolism1, 533–539 (1952).
- J. Wolff, Pharmacol Rev50(1), 89–106 (1998).
- M.A. Greer et al., Environ. Health Perspect. 110(9), 927–937 (2002).
- EPA United States Environmental Protection Agency (2015). http://www.epa.gov/dwstandardsregulations/perchlorate. (Accessed 23 Dec. 2015)
- EPA United States Environmental Protection Agency, Interim Drinking Water Health Advisory for Perchlorate. EPA 822-R-08-025 (2008).
- A. Rübel et al., “Perchlorat in Trink- und Badewasser”, poster contribution at Conference of Ion Analysis (CIA), Berlin Germany, 2011.
- T.J. Scheytt et al., Grundwasser16, 37–43 (2011).
- E. Bloem and K. Panten, J. Verbr. Lebensm.9(4), (2014). doi:10.1007/ s00003-014-0877-9
- EPA United States Environmental Protection Agency. Method EPA 314.0. Determination of perchlorate in drinking water using ion chromatography, Revision 1.0 (USEPA National Exposure Research Laboratory, Office of Groundwater and Drinking Water, Cincinnati, USA, 1999).
- ISO International Organization for Standardization ISO/CD 19340 Water quality. Determination of dissolved perchlorate. Method using ion chromatography (IC) (2016).
- ISO International Organization for Standardization ISO 3696. Water for analytical laboratory use. Specification and test methods (1987).
- ISO International Organization for Standardization ISO 8466-1. Water quality. Calibration and evaluation of analytical methods and estimation of performance characteristics. Part 1: Statistical evaluation of the linear Calibration Function (1990).
- ISO International Organization for Standardization ISO/TS 13530. Water quality guidance on analytical quality control for chemical and physicochemical water analysis (2009).
- R.W. Slingsby and C.A. Pohl, J. Chromatogr. A739, 49–55 (1996).
- DIN (2013) Deutsches Institut für Normung e.V. DIN 2633. Chemische Analytik. Verfahren der Standardaddition. Verfahren, Auswertung, mit CD-ROM.
Maike Anika Seiler holds a B.Eng. in environmental engineering and is currently studying biological and environmental engineering for her Master’s programme.
Detlef Jensen holds a Ph.D. in chemistry, and currently manages the European Support of ion chromatography at Thermo Fisher Scientific.
Udo Neist joined the Hessian State Laboratory about 20 years ago. For the last 10 years he has worked on IC with his current main focus on derivatization techniques.
Ursula Katharina Deister holds a Ph.D. in chemistry and is a professor of environmental chemistry at Hochschule RheinMain (RheinMain University of Applied Sciences, Rüsselsheim, Germany). Prof. Deister works in Rüsselsheim at Hochschule RheinMain.
Franz Schmitz is an analytical chemist with more than 30 years of experience in IC. He works on surrogate parameters (AOX, CIC, TOC, TNb) ion analyses (CFA, IC) and analytical quality control. Franz also participates in standards development
work in DIN, CEN, and ISO and serves as auditor in accreditation projects according
to ISO 17025.
E-mail:Franz.Schmitz@lhl.hessen.de
Website:https://lhl.hessen.de

Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









