Tips & Tricks GPC/SEC: Column Issues with Light Scattering Detectors
The Column
The addition of a light scattering detector dramatically increases the capabilities of gel permeation chromatography/size-exclusion chromatography (GPC/SEC) analysis. However, the complexity of the system also increases. This instalment of Tips & Tricks discusses column issues when working with light scattering detectors.
volgariver/stock.adobe.com

The addition of a light scattering detector dramatically increases the capabilities of gel permeation chromatography/size-exclusion chromatography (GPC/SEC) analysis. However, the complexity of the system also increases. This instalment of Tips & Tricks discusses column issues when working with light scattering detectors.
Detectors to measure the intensity of scattered light in solution are very popular additions to gel permeation chromatography/size-exclusion chromatography (GPC/SEC) instruments because they add true value (1). The light scattering (LS) detector models differ mainly in the position where the scattered light is measured and in the number of simultaneously acquired signals. These detectors should never be confused with evaporative light scattering detectors (ELSDs) (2), which measure scattered light of analyte droplets after mobile phase evaporation.
Light scattering detectors are molar mass sensitive detectors. Their signal response depends on both the concentration and the molar mass. The higher the molar mass and the concentration, the higher the measured signal intensities. The sample itself also plays an important role: the higher the polarizability of the sample (compared
to the solvent), the higher the signal intensity. This sample–solvent parameter is addressed as the refractive index increment, dn/dc.
To obtain the desired results, LS detectors need to be combined with at least one concentration detector, such asa differential refractive index (RI) detector or a UV detector.
In order to gain precise, accurate, and reliable results, impurities that interfere with the LS detector need to be reduced to a minimum.
What are the Challenges When Working with Light Scattering Detectors?
LS detectors respond very sensitively to high molar masses and large sizes and particles, not only from the sample of interest, but also from any contamination in the system.
They are able to detect large analytes at concentrations that are below the detection limit of concentration detectors, such as RI or UV, and therefore using them for analysis is very challenging because they require very clean systems.
An advantage of GPC/SEC-LS compared to batch LS experiments is that the columns can act as a huge filter so that extensive solvent filtration (typical for batch application) can be avoided. On the other hand, columns could unintentionally be a source of contamination.
While the concentration detector shows a stable baseline, that is, it is balanced to the solvent itself, an increased LS baseline level (also referred to as high background) and a noisy LS signal are reported when a molar mass sensitive LS detector is connected for the first time to an existing system or when new columns are installed. Initial bleeding of gel particles eluting from the column may cause a permanent scattering noise level as a result of their high molar mass.
The severity of the problems caused depends on the analytical conditions, such as the stationary and mobile phase used, the sample molar mass, the dn/dc, and the detection angle.
As a rule of thumb:
- The lower the detection angle the greater the likelihood of problems. The 90° angle is not so greatly affected, but low angles are especially prone to a decrease in baseline stability and increase of noise.
- The smaller the dn/dc and the molar mass the lower the signal. Therefore, a higher noise level can be more problematic for low molar mass or low dn/dc samples, while high molar mass and high dn/dc samples can still be analyzed because of a better signalâtoânoise (S/N) ratio.
- Aqueous systems are more complicated than systems operated in organic solvents.
- In organic systems, some organic solvents are more challenging than others.
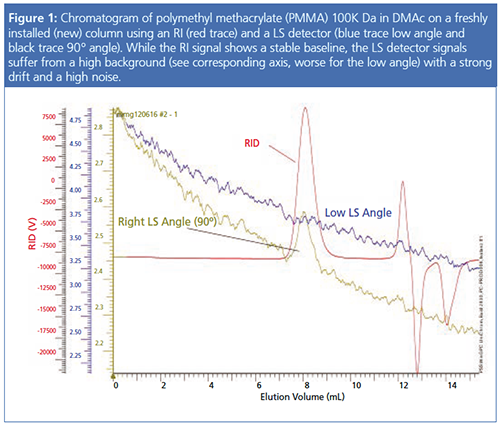
Figure 1 shows the typical problems. While the RI signal is well suited for evaluation, the two light scattering detector signals are not. For the low angle, it is impossible to detect a peak because of the high background, the noise, and the drift.
Influence of the Column Stationary Phase
Two different types of stationary phases are required to cover the wide range of GPC/SEC applications: silica-based materials and polymerâbased materials. Compared to polymer-based columns, silica-based materials have a much higher pressure stability. They are often used for protein separations. Polymerâbased columns are less prone to interaction problems. In addition, they can cover much wider molar mass ranges. As a result of their less steep exclusion and total permeation limits, they can be combined to column sets.
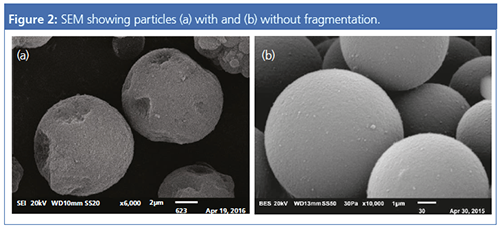
The challenges in GPC/SEC-LS are the same for both column types. The reason for this is the particular structure of the stationary phase. Column packing materials are typically made of particles in the range of <2 to 20 µm. Particles in the nanometre range are formed as well. These nanometreâsized particles and the particle fragments formed during postâpolymerization processes (compare Figure 2) cause the problems described above. They cannot be retained by the frits installed in the columns or by externally installed frits because they are generally much smaller than the mesh size of the frits.
Standardized cleaning and purification after manufacturing, which is sufficient to allow for clean columns generating high quality results with RI, UV, or viscometers, does not ensure LS applicability of the column.
Influence of the Mobile Phase
The mobile phase can affect the contamination dramatically. While the same stationary phase works fine in one solvent, operation in another solvent can be very difficult. This is observed for polymer-based stationary phases comparing tetrahydrofuran (THF) and dimethylformamide/dimethylacetamide (DMF/DMAc).
Silica-based materials behave differently if the mobile phase is changed, for example, from THF to hexafluoroisopropanol (HFIP). While the column works perfectly for the GPC/SEC-LS application in THF, it needs to have an additional cleaning step after switching to HFIP. It is therefore very important that the proper solvent is chosen when the column is ordered from the supplier.
How to Overcome the Problems
To overcome column-related problems in GPC/SEC-LS, one must distinguish between three different scenarios:
a) The problems are always present, independent of the time the columns are in use;
b) The problems occur suddenly, the column used to work perfectly before;
c) The problems occur only when new columns are installed. “Older” columns do not make any problems.
In a) the column used is probably not designed to be compatible for light scattering. In a few cases a filter installed after the columns but before the light scattering detector can be a solution. This filter should be replaced regularly. However, if the filter needs to be exchanged often because of blocking or pressure increase, and mobile phase quality and sample preparation can be eliminated as a problem source, a column replacement is unavoidable. Please note that filters will not help if the impurities are smaller than the mesh size. In addition, filter usage should be monitored carefully because sample parts can be removed by the filter too.
In b) the columns need to be inspected more carefully-either the column bed is damaged or additional impurities elute as a result of solvent or temperature change. The column performance can be tested by measuring the plate count, asymmetry, and resolution (3).
For scenario c), where the columns are generally light scattering-applicable but not ready to use yet, fragments and particle fines can be removed by flushing the columns. In this case, it is recommended to ask for a cleaning or purification process, if possible, from the supplier. While doing the purification step, the column should not be connected to any type of detector to avoid detector contamination. However, this process may take several column volumes to remove the fine parts from the huge surface of the macroporous column material.
Some column manufacturers have responded to this tedious time-consuming challenge by offering stationary phases specifically optimized for light scattering detection. These columns are pretreated to reach the steady state faster and to show less noise so that they are ready to use directly after installation. During the pretreatment, the noise level goes down constantly and the S/N increases with reduced noise level. This is shown in Figure 3. Here the same sample has been injected on the same column, which is halfway through the cleaning process. The baseline level of the light scattering detector signals has decreased significantly (compare the two right y-axes in Figure 1) and the noise has also been reduced.
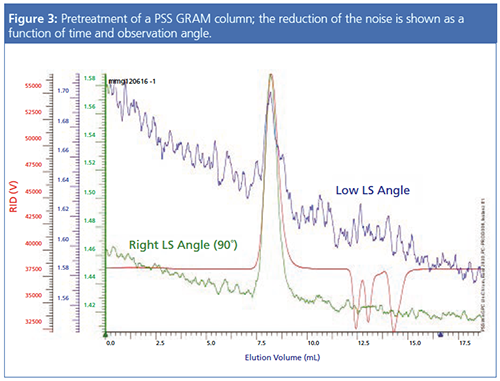
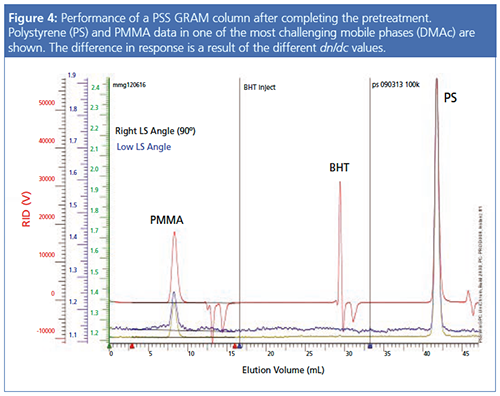
Figure 4 shows the final result after the pretreatment. The noise level has significantly decreased for both LS angles. The polymer signals correspond to a polymethyl methacrylate (PMMA) and a polystyrene (PS) of the same molar mass in one of the most challenging mobile phases, DMAc. The middle injection shows butylated hydroxytoluene (BHT) injected for column testing. The PS signal has a higher intensity because of the higher dn/dc compared to PMMA.
Summary
- While a column can be sufficiently clean for a concentration detector, this may not be the case for LS detectors.
- Light scattering detectors respond to low concentration and large size contaminants, resulting in increased baselines and higher noise for systems that are not ultra-clean.
- Small nanometre-sized particles and particle fragments need to be removed for both porous polymer- and porous silica-based particles.
- Pretreated light scattering columns available from the supplier show a low noise level after a short period of time.
References
- D. Held and P. Kilz, The Column5(4), 28–32 (2009).
- D. Held, The Column9(18), 2–5 (2013).
- F. Gores and D. Held, The Column10(14), 7–10 (2014).
Thorsten Hofe studied chemistry in Mainz, Germany, and Toronto, Canada. He is the head of the PSS production department (columns, reference materials, and polymer synthesis). He is also involved in education and customer training.
Moritz Susewind studied inorganic chemistry in Mainz and works in the PSS production department. He is responsible for the development of new and improved stationary phases.
E-mail: DHeld@pss-polymer.comWebsite:www.pss-polymer.com

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Rethinking Chromatography Workflows with AI and Machine Learning
April 1st 2025Interest in applying artificial intelligence (AI) and machine learning (ML) to chromatography is greater than ever. In this article, we discuss data-related barriers to accomplishing this goal and how rethinking chromatography data systems can overcome them.
Influence of Concentration in Conventional GPC/SEC and Advanced Detection GPC/SEC
March 21st 2025Sample concentration is a parameter that can influence the quality of gel permeation chromatography/size-exclusion chromatography (GPC/SEC) separations and the obtained results. Understanding this influence can help to support the development of reliable GPC/SEC methods.