The LCGC Blog: Critical Evaluation of Chromatography Methods (II)—Evaluating Gas Chromatography Methods
In my October blog, I introduced the concept of critical evaluation of chromatographic methods as a skill that every analytical chemist should possess. The ability to predict issues and highlight unusual method parameters, before ever entering the laboratory, allows you to be alert for potential issues with a separation, or to change the method (where applicable) prior to initiating any experimentation. These detective skills, when applied in retrospect, are also invaluable for troubleshooting problems with the chromatographic separation or quantitative results.
As with any profession, there really is no substitute for a lifetime of experience and the “school of hard knocks” is a successful school indeed, however, the intention with this series is to condense some of these invaluable lessons into bite-sized articles, which will help you to quickly gain the ability to critically evaluate your methods and link practical issues with methodological “quirks.”
This time we turn our attention to a gas chromatography (GC) method that I recently came across that was producing less than ideal results. I asked not to know anything of the problems, rather that I was given the method to study in the hope that I could predict some of the issues and hopefully guide the analyst to a better-performing method.
The method, outlined below, is for the analysis of a suite of barbiturate compounds in an extract from physiological matrices. While there were a number of issues with the extraction method, we will consider only the chromatographic aspects here, leaving the evaluation of extraction methods for a future issue.
Column: 5% phenyl polydimethylsiloxane, 30 m x 0.25 mm, 0.25 µm
Carrier: Hydrogen at 60 cm/s, measured at 60 °C
Oven: 60 °C, 60–150 °C at 25 °C/min, 170–300 °C at 10 °C/min
Injection: Splitless, 250 °C, 1 min purge time
Sample: 2 µL of extract (isopropyl alcohol)
Detector: MS, 250 °C transfer line full scan at m/z 40–270
FID, 300 °C, air 450 mL/min, hydrogen 30 mL/min, helium makeup
50 mL/min.
Capillary flow splitter with makeup to balance retention times
between both detectors
So, where to start with the critical evaluation of a GC method such as this?
I always like to know the chemistry of the analytes, so Figure 1 shows analyte structures for some of the target analytes in this method.
Figure 1: Selected barbiturate analytes from the analytical method under consideration

The barbiturate structures, with their multiple aldehyde and amine groups, would indicate that these relatively small analyte molecules would be reasonably polar in nature. The LogD value of barbital, for example, is 0.6 at pH 7, falling to 0.1 at pH 8. The GC column stationary-phase choice of 5% phenyl polydimethylsiloxane, however, if separation problems occur a more polar phase such as the 35% phenyl polydimethylsiloxane, could be considered.
The analysis of structurally related target compounds often requires the use of longer and narrower bore GC columns, especially when using apolar phases, which tend to separate on analyte volatility. Where boiling points do not differ greatly, a stationary phase that can chemically discriminate between analytes should be considered. This seems to be the case for our analytes above. Once in the laboratory, if we discover that there is poor resolution between analytes, apart from selecting a more highly polar stationary phase as discussed above, we may need to move to a longer (60 m) column, or to reducing the column internal diameter to drive the separation through improved efficiency rather than chemical means. Of course, a longer column will extend the analysis time, and the use of 0.18 mm internal diameter columns requires careful planning and reduced extra column volumes within the GC system. Because the barbiturate analytes are not highly volatile, the relatively thin stationary phase film should be satisfactory.
For more information on GC column phase selection – see the following link;
https://www.crawfordscientific.com/chromatography-blog/post/gc-column-selection
Using hydrogen carrier gas with mass spectrometry (MS) detection should always be considered as a potential issue, due to the vacuum at the outlet of the GC column leading to very low injector head pressure requirements. One should consider the gas chromatograph head pressure requirements, because very low values may lead to irreproducible retention times, separation variability, or even an inability for the instrument to attain or maintain a pressure setpoint. While 60 cm/s is a reasonable linear velocity for hydrogen as a carrier gas with the column dimensions used in the method, due to the fact that we have a vacuum at the column outlet (meaning the MS detector), the head pressure associated with this linear velocity under the method conditions is only around 2 psi, which may be difficult for the instrument gas pressure control systems to deliver in a reliable fashion, even though the quality and reliability of gas pressure or flow control units are very high in modern instruments.
Figure 2: Pressure and flow calculator used to assess the GC method under consideration
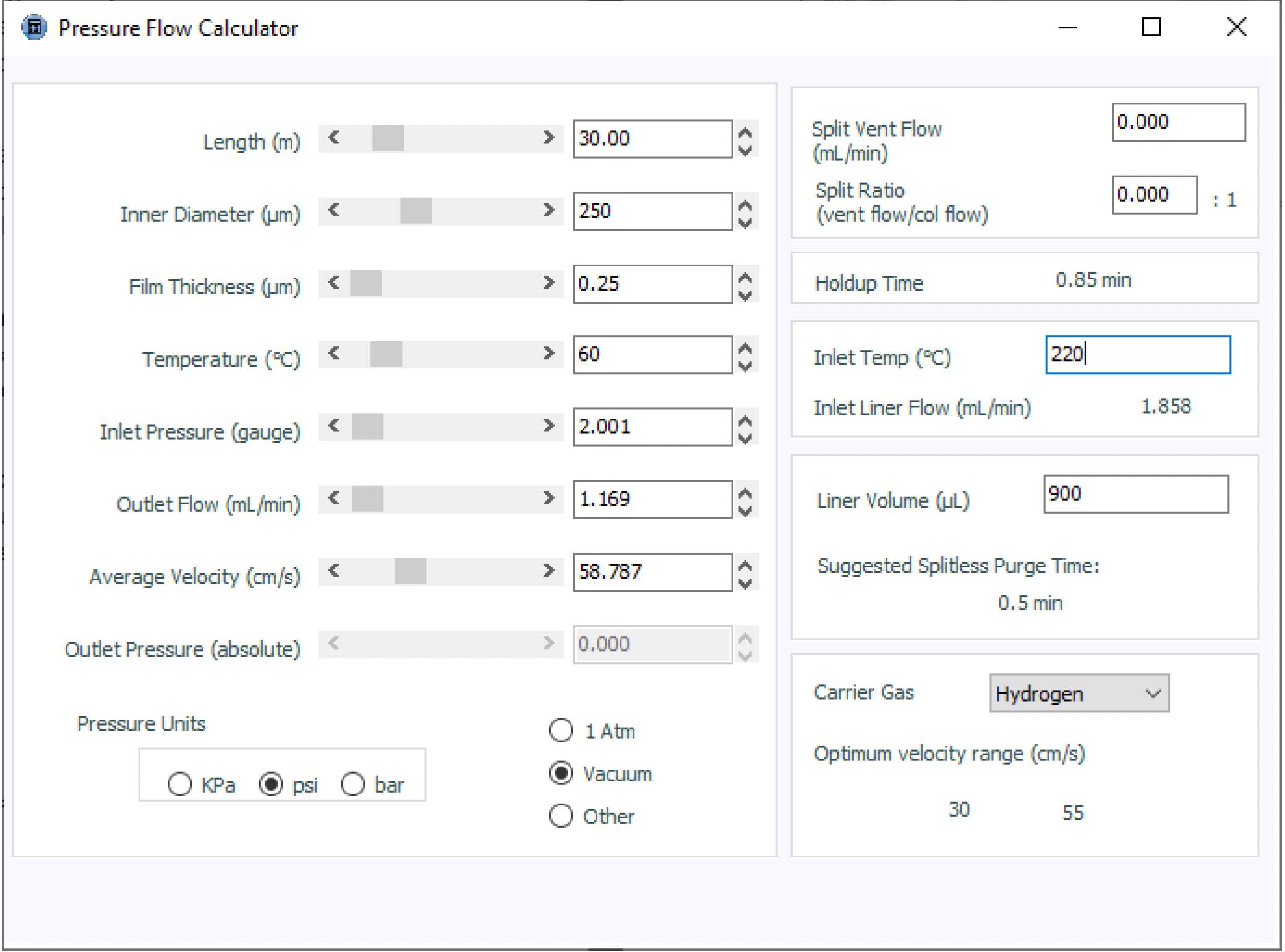
I used a manufacturer's pressure/flow calculator to calculate the pressure required to achieve the desired linear velocity of the carrier gas under the method conditions ahead of even venturing into the laboratory. These tools are excellent for our critical evaluation toolkit, in order to evaluate and anticipate problems such as those described above. The use of wider-bore capillary columns with low linear velocities, the use of hydrogen as a carrier gas, and the fact that MS detectors present us with a vacuum at the column outlet can all give rise to potential problems with low carrier gas outlet pressures, all of which can be investigated ahead of any practical work, using the type or pressure/flow calculator shown in Figure 2. One further parameter worthy of note is the outlet flow, which is shown in Figure 2. In this case, the flow is actually split between the mass spectrometer and an FID detector using a capillary flow splitting device, so we cannot assume that the full 1.1 mL/min of carrier gas reaches the spectrometer. However, this is not always the case, and it is worthwhile to “predict” the outlet flow coming from the analytical column to ensure that it does not exceed the maximum for the spectrometer in use. Even though modern vacuum pumps can often withstand gas flows of 2 or even 4 mL/min of carrier gas flow (check the manufacturer’s flow or pressure limits for your particular instrument), it is always good practice to check the column outlet flow rate under your particular experimental combination of carrier gas type, pressure, and column dimensions. Exceeding the carrier gas flow limits will cause a noticeable reduction in the performance of the detector, especially in terms of the analyte ionization characteristics (spectral appearance or analytical sensitivity), and may even cause the instrument to shut down. One should also bear in mind that the use of hydrogen carrier may require adjustments to the MS instrument hardware and may also result in changes to the spectra of certain analyte types. For further information on the use of hydrogen with MS detectors, see the following links:
https://www.crawfordscientific.com/chromatography-blog/post/hydrogen-carrier-gas-ms-detection
When using splitless injection, it is typical to start the oven temperature program at around 20 oC below the boiling point of the sample solvent, to ensure good thermal focusing of the analyte at the head of the analytical column, and therefore sharp, rather than broad or split peaks. In this case, the solvent is isopropyl alcohol with a boiling point of around 82 oC and an oven starting temperature of 60 oC seems appropriate, and we would assume that certainly, the earlier eluting peaks within the chromatogram should be “sharp” from a chromatographic perspective. This so-called “solvent-focusing” phenomenon applies mainly to earlier eluting species because it is assumed that the more volatile analytes will remain associated with the injection solvent vapor when transitioning from the inlet into the analytical column. If the starting oven temperature were any higher, the solvent containing the analyte molecules would be unable to condense onto the inner walls of the capillary column, which is the basis of the solvent-focusing effect, and peak shape broadening or deformation could occur. It should also be noted that it is usual practice to hold the initial oven temperature to allow optimum peak focusing through both thermal and solvent effects. In this method, there is no initial hold time, and this can potentially lead to peak shape deformation (typically peak splitting/tailing) and issues with peak area reproducibility. One should consider the introduction of an isothermal period that initially matches the purge time (see below) and which can be empirically optimized by extending or shortening the hold time, to overcome any peak shape issues.
There are two further important considerations when investigating potential issues with a new method that uses splitless injection: the injection volume, as well as the splitless or purge time associated with the injection phase. Splitless injection, as you may be aware, is used when we need to introduce all, or the vast majority, of our sample into the GC column in order to obtain the required sensitivity when analytes are present at very low concentrations. To achieve this, during the first part of the injection phase, the split valve is closed and all of the sample vapor produced in the inlet is passed to the column. After a pre-defined time, the split valve is opened, and any residual solvent/sample vapors are vented from the inlet to avoid large/tailing solvent peaks and rising baselines as well as to protect the inlet from contamination. It is important that during the splitless phase of the analysis, the volume of sample vapors generated does not exceed the available volume within the inlet (predominantly defined by the internal volume of the inlet liner). If the inlet volume is exceeded, a process colloquially known as “backflash” can occur. Backflash may lead to deposition of analyte within the gas supply and outlet lines leading into and out of the inlet, ultimately risking carryover and problems with quantitative reproducibility.
Figure 3: Vapor volume calculator used to assess the possibility of backflash during injection
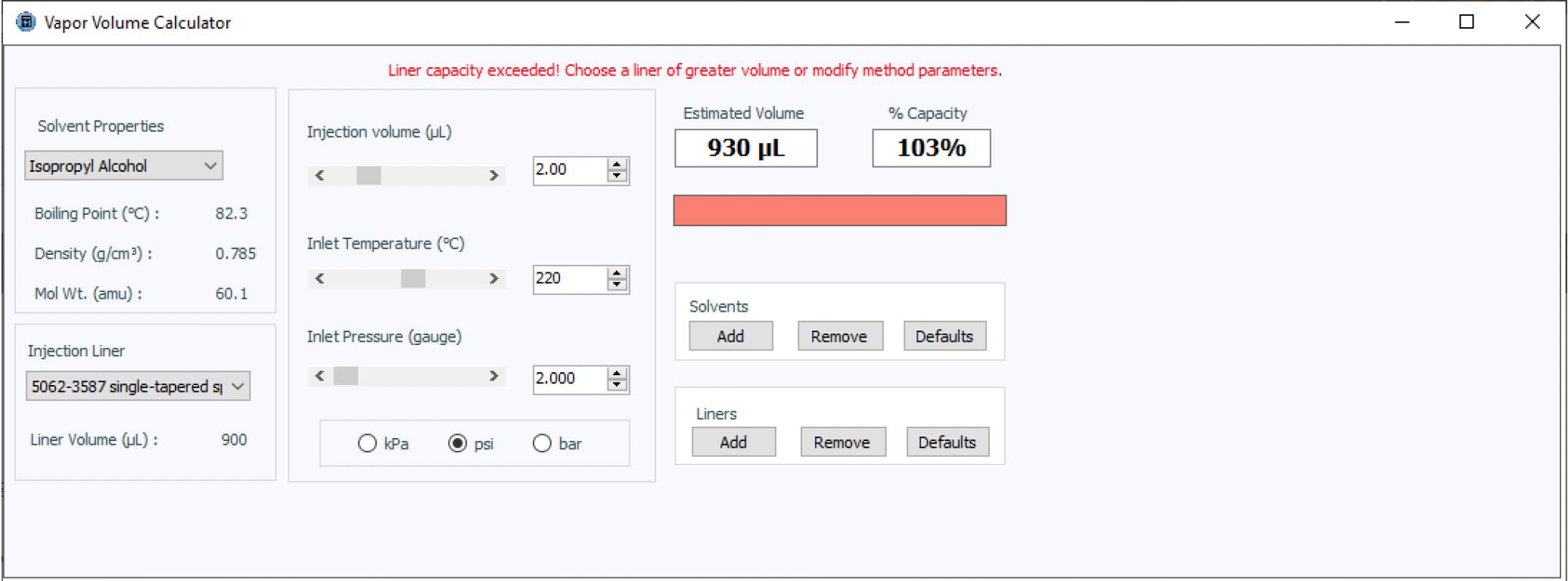
Again, I have used a helpful tool to predict the vapor volume created under the conditions used in our method. Figure 3 shows that the 2-mL injection under the inlet conditions selected is dangerously close to the total volume of the liner chosen. If issues with carry-over are encountered when implementing the method, one might consider reducing the injected volume, if sensitivity requirements can still be met. Alternatively, one could also consider a pressure-pulsed injection, where the inlet pressure is raised during the injection to constrict the expansion of the sample vapor. This will avoid backflashing the solvent vapor, while retaining the 2-mL sample injection size.
For a more comprehensive treatment of optimizing splitless injection conditions with pressure pulsed injection, see these links:
https://www.crawfordscientific.com/chromatography-blog/post/pulsed-pressure-splitless-gc-inject
The purge, or spiltless time corresponds to the time at which the split vent is opened to release any lingering vapors and needs to be optimized empirically. Typically, one would begin with shorter purge time (30 s would be typical), and the purge time lengthened until the peak area of analytes stabilizes. Too short a purge time leads to analytes that have not passed into the column being lost from the split line. Typically, the analyte peak area will stabilize and remain constant, and the purge time should be increased in 10– 15 s increments until the peak area stabilizes and the purge time set to the value that corresponds to the second time increment where peak area remains stable. The 1-min purge time within the method seems reasonable. However, if the solvent peak tails or the baseline is noisy and rises dramatically throughout the analysis, one may consider reducing the purge time.
Without seeing a chromatogram, it is very difficult to predict the suitability of the temperature program gradient, however, when analytes are very similar in chemistry (homologs and such), the rule of thumb is that lower ramp rates tend to work better. The 25 oC ramp rate at the start of the analysis should be carefully considered if problems with selectivity/resolution are encountered. However, as the gradient program in our method slows to 10 oC per min from 170 oC, then I suspect that any problematic analyte peak pairs occur from 4 min onwards within the chromatogram, and the rapid initial program is merely used to reduce overall analysis time. If one finds a need to further optimize the oven temperature gradient, then many good tips can be found at these links:
https://www.crawfordscientific.com/chromatography-blog/post/gc-temperature-program-development
In terms of the flame ionization detector (FID) parameters, the absolute values for temperature and gas-flow rates will vary by manufacturer, again, though we can highlight some general principles. It is usual to keep the detector temperature elevated above the final oven temperature, to prevent condensation effects. One should consider raising the detector temperature from 300 oC to 325 or 350 oC to prevent water condensation in the detector, which reduces sensitivity, and to reduce the risk of analyte deposition within the detector body. The gas-flow settings within the method seem reasonable, although most methods tend to have similar gas flow for the fuel (hydrogen) and make-up (helium) gas flows, typically because they have not been optimized to obtain the best sensitivity. In our example, the make-up flow is higher than the fuel gas flow, but this may well be because the method has been optimized. If not, we should consider reducing the make-up gas flow. Typically the air-to-fuel gas ratio would be around 10:1, and in the method cited, the ratio is a little higher than this. Again, if sensitivity needs to be improved, one might consider lowering the airflow rate or slightly increasing the fuel gas flow, however, these recommended settings are somewhat manufacturer dependant.
The MSD transfer line is a heated line that carries the column effluent from the oven to the ion source within the detector. The temperature of the transfer line should be set to avoid condensation of analytes within the heated region, which may result in reduced method sensitivity and carry-over. It is recommended that the transfer line temperature should not be more than 20 oC below the oven temperature and therefore, in our method, we should consider raising the transfer line temperature to at least 280 oC. No further details are given regarding the other MS parameter settings, which is an omission in the method specification. For brevity, we will consider critical evaluation of MS detector settings in a future blog entry.
Who would have thought that there was so much to discuss from a few simple GC method settings! The skill in critical evaluation of methods is to be prepared, almost to predict issues before they occur. In reality, this method may work perfectly well in practice, however undertaking this short evaluation exercise will allow us to prepare for any problems, and to prioritize the adjustments we might make if specific issues arise.
Tony Taylor

Tony Taylor is the Chief Scientific Officer of Arch Sciences Group and the Technical Director of CHROMacademy. His background is in pharmaceutical R&D and polymer chemistry, but he has spent the past 20 years in training and consulting, working with Crawford Scientific Group clients to ensure they attain the very best analytical science possible. He has trained and consulted with thousands of analytical chemists globally and is passionate about professional development in separation science, developing CHROMacademy as a means to provide high-quality online education to analytical chemists. His current research interests include HPLC column selectivity codification, advanced automated sample preparation, and LC–MS and GC–MS for materials characterization, especially in the field of extractables and leachables analysis.
New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





