Strategies for the Quantification of Endogenously Present Small Molecules in Biological Samples
LCGC Asia Pacific
The main objective of this review article is to provide a clear summary of the different methods that can be used to quantify endogenous small molecules. Because of the increased use of mass spectrometry (MS) in the field of bioanalysis, a special focus will be placed on quantification by liquid chromatography (LC)–MS. Practical recommendations to face this bioanalytical challenge, in particular in terms of method validation, will also be provided.
The quantification of endogenously present compounds in biological samples demands appropriately validated methods, in particular because increasing research efforts are aimed at studying the impact of such compounds on human health and disease. International regulatory agencies, such as the Food and Drug Administration (FDA) and the European Medicine Agency (EMA), have published a vast number of guidelines concerning bioanalytical method validation over recent decades. Compared with the quantification of exogenous compounds, these guidelines have remained rather vague and unclear when it comes to the quantification of endogenous compounds. Nonetheless, the continuous expansion of studies devoted to the search for endogenous disease markers in the human metabolome has incited the regulatory bodies to include endogenous compounds in a draft of an updated International Conference on Harmonization (ICH) guideline on bioanalytical method validation. In light of these recent developments, the main objective of this review article is to provide a clear summary of the different methods that can be used to quantify endogenous small molecules. Because of the increased use of mass spectrometry (MS) in the field of bioanalysis, a special focus will be placed on quantification by liquid chromatography (LC)–MS. Practical recommendations to face this bioanalytical challenge, in particular in terms of method validation, will also be provided.
Endogenous analytes serve as important upstream indicators and informative sources for downstream biological processes (1). Apart from providing a platform for the in-depth understanding of the underlying molecular mechanism of a disease, metabolites might also play key roles as “biomarkers” in the early diagnosis and prognosis of diseases. The surge in high resolution and sensitive mass spectrometry (MS) instruments has tremendously propelled the realm of metabolomics and, hence, the human metabolome has received increased attention as a major source of information related to health and disease.
Nonetheless, a reliable quantification, in terms of accuracy and precision, is hampered by the lack of a true blank matrix, that is, a matrix without the analyte of interest, in order to prepare matrix-matched calibrators (2). The use of matrixâmatched calibrators is recommended in the analysis of biological samples, particularly when a mass spectrometer is used. This is because matrix effects (MEs) are likely to occur in the ionization source that might result in false negative and false positive diagnostics (3,4,5). An additional challenge lies in the absence of samples with a priori known quantities of the compound of interest, which are required to check for the accuracy of the method and the determination of the quantitation limit. Even though the most recent Food and Drug Administration (FDA) guidelines (2018) on bioanalytical method validation address the challenge related to the quantification of endogenous compounds, they merely provide some general advice on the use of analyte-free matrix and the need to evaluate parallelism (6). The Japanese Ministry of Health, Labour, and Welfare (MHLW) recognizes the use of a surrogate matrix on condition that its validity has been shown, but it does not specify any further practicalities (7). The European Medicine Agency (EMA), on the other hand, does not provide any considerations on endogenous compounds in its guidelines (8). Nonetheless, very recently, a joint effort of these three regulatory bodies in the International Conference on Harmonization (ICH) has resulted in a draft version of bioanalytical guidelines, which now addresses the issue of endogenous compounds (9). The respective regulatory agencies are accepting comments until September 2019 before its formal implementation.
Over the years, three major strategies have been proposed to quantify endogenous compounds: (i) the standard addition method (SAM), (ii) quantification in a surrogate matrix, and (iii) quantification with a surrogate analyte. As well as these, the use of background subtraction has also been suggested. In the latter, the calibration curve is created by spiking authentic matrix with increasing concentrations of the compound of interest. The resulting response curve is subsequently corrected for the endogenous (background) signal of the compound of interest in the original matrix. Since the quantification limit is confined by the endogenous levels and is consequently larger than in other methods, the use of background subtraction has remained limited (10). Nonetheless, correction of the resulting peak area for background signal can serve as a viable way to deal with the insufficient removal of the compound from the matrix by, for instance, charcoal treatment (11). Each of the approaches mentioned is associated with practical challenges, advantages, and drawbacks, which are discussed in the first part of this review. In the second part, further potential issues related to the validation of the analytical method will be addressed, including the determination of linearity, parallelism, accuracy, matrix effects, and the limit of quantification.
Quantification Strategies
Standard Addition Method: The standard addition method (SAM) is recommended when no internal standards (IS) are available to compensate for pronounced matrix effects. In particular in multicomponent analysis, finding an adequate IS for each analyte is not always possible. The standard addition method comprises the addition of a reference standard of the analyte of interest at increasing concentration levels into multiple aliquots derived from the same biological sample in which the endogenous concentration of the analyte has to be determined. One aliquot remains unspiked. After analysis, the detector response is plotted against the added concentration and the endogenous concentration is derived from the negative intersection with the x-axis (Figure 1). As an alternative, the concentration can be plotted against the detector response and the endogenous concentration can be obtained from the intersection with the y-axis, which can be immediately derived from the regression equation. This is referred to as the reversed-axis method (Figure 1) (12,13).
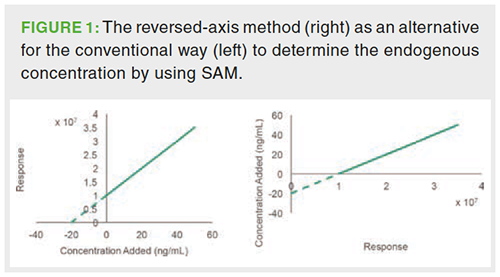
SAM considers matrix effects by relying on the assumption that all concentration levels-both endogenous and spiked-experience a proportional ion enhancement or suppression effect because each aliquot contains the same amount of coeluting matrix compounds. As a new calibration line is created for each individual sample, the method also considers interâindividual differences in matrix effects. The use of SAM has remained rather limited in the liquid chromatography–mass spectrometry (LC–MS) analysis of biological samples because matrix effects are mostly compensated by the use of an appropriate IS (14) (see section “Surrogate Matrix Approach”).
The use of extrapolation presumes that the relationship between the concentration and the response remains unchanged outside the linear range. Therefore, the spiked concentration range should be as close as possible to the endogenous levels. The EU guideline on pesticide analysis, a field where SAM is more commonplace, prescribes that the added amounts should be within one half and five times the endogenous concentration (15). However, this requires information on the endogenous concentration levels, which is, of course, the question of interest. To deal with this, an explorative study needs to be conducted. One possible method regards the estimation of these levels using an external calibration curve created in solvent (16). Nevertheless, it is doubtful whether the concentration obtained by this method can serve as a trustworthy prediction because of the occurrence of matrix effects, which, moreover, might also vary between samples. As an alternative, a certain number of samples can be spiked at a wide range of concentration levels. After choosing an appropriate calibration model to fit the data, the curve can be extrapolated to a tentative value for which the subsequent spiking levels are selected, at for instance 0.5, 1, and 2 times the tentative value. The remaining spiking levels are omitted and the curve is again extrapolated to zero response. Once the average expected endogenous concentration is established, the remaining samples can be spiked with a lower number of spiking levels, which cover approximately 0.5, 1, and 2 times the expected level. This approach was used by Olesti et al. (16) and resulted in values that did not differ more than 15% from those obtained by employing the surrogate matrix approach with the inclusion of an IS (see section “Surrogate Matrix Approach”).
Even though the use of an IS is not required to compensate for matrix effects, recent work by Hewavitharana et al. (2018) has shown that the addition of an internal standard can successfully correct for procedural errors and variations in instrumental response (13). They showed that the IS can even be used to correct for other compounds, provided that the response of the internal standard is not influenced by its unlabelled counterpart. This method was proven to be superior to the use of an IS as surrogate analyte.
Surrogate Matrix Approach: The most widely adopted way to quantify endogenous components in biological samples is by using a surrogate matrix. This approach is often called isotope dilution and involves the use of the authentic analyte in an analyte-free matrix in order to prepare the calibration standards. The endogenous concentration is then simply calculated from the calibration curve by interpolation. Surrogate matrices cover a broad range of alternatives for the original matrix going from its most simple form, a solvent or buffer, to more complex alternatives including so-called stripped matrices in which the analyte has been removed from the matrix (17–20).
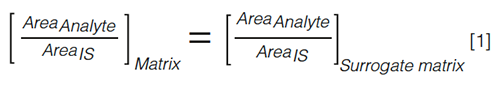
Even though the method is simple and straightforward, it is important to recognize that the matrix effects exercised on the analyte might differ considerably in the authentic and surrogate matrix. Even when the samples are subjected to an extensive sample clean-up, such as solid-phase extraction (SPE), matrix effects might still be pronounced (5,21). Therefore, an IS can be added to both the calibrators and the samples at a fixed concentration. The general idea is that the IS experiences the same matrix effect as the analyte of interest and, hence, their peak areas are affected to the same degree. Regardless of any matrix effect, the analyte to IS peak ratio will thus remain unaffected. For a given concentration, the following equation (equation 1) then applies:
In order to experience the same matrix effect, the natural analyte and its IS should have the same retention time and similar physicochemical properties. A stable isotopically labelled internal standard (SIL-IS) is considered the best option due to its structural relatedness with the nonlabelled analyte. The difference in mass generated by isotopic labelling provides the option to differentiate the structural analogues using MS. However, given the natural abundance of isotopes, the SILâIS should differ at least three mass units from its nonlabelled analogue in order to minimize spectral overlap (22).
Despite its structural similarity, a SIL-IS does not always guarantee successful compensation for the matrix effect experienced by a target compound. The replacement of a carbon-hydrogen bond by a carbon-deuterium bond, for example, might decrease the polarity of the molecule and affect its retention time. This phenomenon is known as the isotope effect and can be circumvented by employing 13C or 15N labelled internal standards or by altering the chromatographic conditions (23,24). Regarding which concentration of IS to add, it is vital to investigate the influence of the concentration of both IS and target analyte on each other’s ionization signal in advance. A suitable IS concentration should be selected to keep the product of the peak area ratio (analyte/IS) and the inverse of the analyte concentration (equation 2) constant across the entire linear range (25):
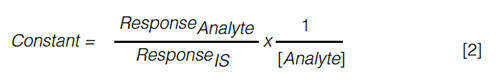
Apart from compensating for matrix effects, the use of SIL-ISs offers other advantages. It considers inter-individual differences since the response ratios at a certain concentration will not differ from sample to sample, even though the matrix effect might be present to a different extent (21). A SIL-IS can also compensate for other variable parameters that might affect accurate and precise quantification, such as procedural errors during sample clean-up (21,25,26). Nevertheless, it is important to always assess whether analyte and SIL-IS possess equal extraction recoveries (27–29).
Notwithstanding these advantages, the choice for a SIL-IS might be restrained as a result of economic reasons or commercial unavailability, particularly in multicomponent analysis. In this case, the standard addition method might be a viable alternative.
Surrogate Analyte Approach: In this approach, the calibration curve is created in authentic matrix by using a surrogate analyte possessing an identical ionization behaviour as the naturally occurring compound. This is a stringent requirement as both compounds should produce identical peak areas at each concentration level to generate accurate results. For this reason, a response factor (RF), expressing the difference in peak area at the same concentration, is generally calculated (equation 3) (28,30–31). Because of the endogenous nature of the analyte, the RF can only be calculated in neat solution. The RF should ideally be equal to 1 and can be optimized by altering the collision energy in the MS instrument (28). Alternatively, a correction factor reflecting the average RF at different physiologically relevant concentration levels should be included in the final calculation (equation 4) (17,31). The RF is sometimes omitted in the final calculation of the endogenous concentration if the difference in response is not greater than 5% (28,32). The RF should be checked regularly after optimization because changes in ionization efficiency might occur, for example,after shutdown of the instrument (30).

Regarding the choice of the surrogate analyte, a labelled analogue offers the best option given its structural similarity with its natural counterpart and, hence, similar ionization behaviour. Moreover, since the surrogate analyte does not consider matrix effects, an IS, correcting for the matrix effects experienced by the natural as well as the surrogate analyte, must be added as well. This triangular relationship demands a strong compatibility between natural analyte, surrogate analyte, and IS for which labelled analogues are the best choice. In this case, the mass of all the analogues should differ sufficiently to prevent overlap of their respective isotopic pattern. The endogenous concentration is calculated in accordance with the following formula:

in which m represents the slope of the calibration curve, b its y-intercept, and RF the average response factor.
Validation
Regression Analysis and Parallelism: The calibration range should comprise a physiologically relevant range wherein at least six equally distributed concentration levels are included (33,8). Although EMA and FDA do not compel the replicate analysis of each calibrator, it is advised to perform at least duplicate analysis at each level (6,8,34).
The choice of an appropriate regression model is necessary to ensure accurate results. To fit the data to a linear curve, the ordinary least squares regression (OLS) method is the simplest approach in bioanalysis. The OLS method assumes that the response variables have equal variances across the entire concentration range, often referred to as homoscedasticity (35). To verify this, the residuals can be plotted against the concentration (34). In case of homoscedasticity, the residuals will be homogenously distributed around the x-axis. Another fast approach comprises the computation of the F-value, which represents the ratio of the highest to the lowest variance (36–38). This calculated F-value is compared against the critical F-value to evaluate whether the assumption is violated or not.
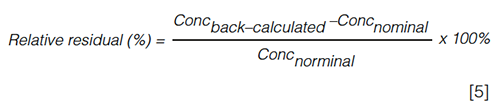
In case of unequal variances, the number of calibration points can be decreased because higher variances are often observed at higher concentrations. This can only occur at the cost of a reduction of the interpolation range (36). When SAM is used, the highest spiked values can be eliminated, provided that a sufficient amount of calibration points remain to assess linearity. Alternatively, a weighed linear regression (WLS) model can be applied (37). In bioanalysis, the following empirical weighing factors are commonly used: 1/x, 1/x², 1/y, and 1/y². To verify which weighing factor is appropriate, the back-calculated concentrations of the calibrators should be compared with the nominal values. The relative residual (equation 5) should be within 15% for at least 75% of the calibration levels (6,8,16,37):
If linear regression models are not sufficient to fit the data across the desired concentration range, polynomial regression models can be considered. Note that the addition of an increasing number of independent variables will automatically result in an increased coefficient of determination (R²). Even though it is often used as a criterion to gauge the goodness of fit of the calibrator data, an increased R²-value might also be the result of overfitting and, hence, does not necessarily imply a higher predictive power. Therefore, other formal tests to assess whether an increase in prediction parameters improves the fitting are more suitable, such as the lack-of-fit test (LOF) or Mandel’s test (39–41). In accordance with Occam’s razor, the simplest model describing the relationship is always preferred (6).
Because the detector response might be different in matrix and solvent, international guidelines advocate the use of matrixâmatched calibrators. Strictly speaking, the standard addition method encompasses the use of matrixâmatched calibrators because a calibration curve is created in each individual sample (13). Nonetheless, it is necessary to be aware that SAM assumes a linear relationship outside the calibration range and as aforementioned, it is for this reason required to evaluate linearity as close as possible to the endogenous concentration value.
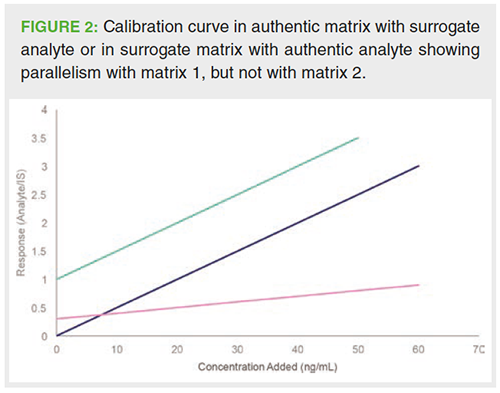
The surrogate analyte approach involves the use of authentic matrix to create the calibration curve. However, it must still be verified whether matrix effects experienced by the authentic and surrogate analyte are equal. This can be examined by showing so-called parallelism (28,42). From a practical point of view, a matrix spiked with authentic analyte at increasing levels is compared with the same matrix spiked with increasing levels of the surrogate analyte (Figure 2). The IS is added at a fixed concentration level each time to correct the peak area of authentic and surrogate analyte. If the obtained slopes of the calibration curves do not differ significantly from each other, parallelism has been demonstrated. Significance can be traced by a formal t-test (43).
In contrast with SAM and the surrogate analyte approach, the surrogate matrix approach does not involve the creation of the calibration curve in authentic matrix. In this case, demonstrating parallelism between the authentic and surrogate matrix is necessary to ensure the valid use of a surrogate matrix (43,44). Both authentic and surrogate matrix are for this purpose spiked at increasing levels of the analyte and at a fixed level of the IS. The regression curves are compared as described above (Figure 2).
Accuracy: Accuracy is a measure to describe the trueness with which the concentration is determined in samples (quality controls [QCs]) containing known amounts of analyte, that is, the nominal amount. The concentration is determined against the calibration curve and the accuracy value is expressed as the ratio of the determined value to the nominal value. The accepted deviation from the nominal value is set at ± 20% for the LLOQ and ± 15% for higher levels. However, as the endogenous concentration is unknown, the accuracy of the method cannot be assessed in this way for endogenous compounds. This hiatus can be bypassed by using equation 6, in which CM represents the measured concentration upon spiking, C0 the measured basal concentration level, and Cs the nominal spiked concentration (Figure 3) (45):
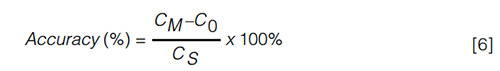
Both CM and C0 are determined using either one of the suggested methods in the ”Quantification Strategies” section.
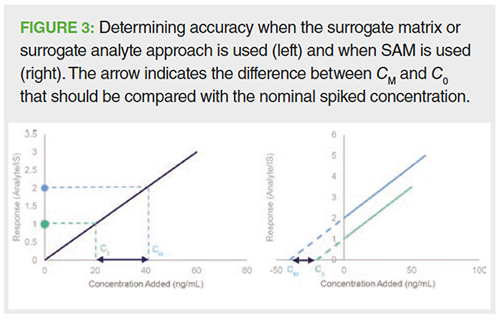
Since the physiologically relevant range might be surpassed by spiking the authentic matrix, the draft version of the ICH bioanalytical guidelines recommends the use of QC samples with the lowest endogenous concentration (9). Besides stating that the spiked concentration levels should be statistically different from the endogenous concentration, it does not provide any further requirements regarding spiking concentrations. However, it should be recognized that spiking outside a physiologically relevant range might undermine the actual accuracy of the method. Therefore, spiking levels are suggested to not exceed four times the expected endogenous concentration (46).
The determination of accuracy through spiking might also enable to demonstrate the validity of the utilization of the surrogate matrix or surrogate analyte for quantification purposes as accuracy values within predefined limits justify their usage (9,17,32,42).
Contrary to SAM and the surrogate matrix approach, the surrogate analyte approach enables the accuracy of the method below the natural endogenous level to be monitored by using the surrogate analyte to prepare QCs (30).
Matrix Effect: The matrix effect can be calculated in multiple ways. EMA suggests the calculation of a matrix factor (MF) (8). This parameter compares the response of the analyte spiked at the same concentration level in authentic matrix and in neat solution. In case the sample undergoes a pretreatment step, the analyte is spiked in the extracted matrix to differentiate the matrix factor from recovery (47,48). The method is often referred to as the post-extraction spike method (48). To circumvent interfering responses arising from basal analyte levels, the peak area of the unspiked matrix is subtracted from the peak area of the spiked matrix (equation 7) (29,47):

An MF < 1 indicates ion suppression and an MF > 1 indicates ion enhancement with respect to the response of the analyte in neat solution.
The IS should experience the same matrix effect as the analyte of interest in order to function as an appropriate IS. The matrix factor of the IS can be calculated without any correction for endogenous levels (equation 8) (8,17,29).
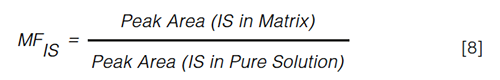
By dividing the MF of the analyte with the MF of the IS, the IS-normalized MF is calculated. In spite of the absence of any limits provided by the regulatory agencies, the normalized MF should be as close to unity as possible. The inter-individual variability of the IS-normalized MF, often referred to as the relative matrix effect, should be assessed by analyzing a minimum of six different samples. The variability of the matrix effect among the samples is expressed in terms of covariance and should not exceed the limit of 15% (8). More stringent cut-off values (CV < 5%) have, however, also been put forward (48,49).
Alternatively, the matrix effect can be assessed by comparing the slopes of a standard addition curve in matrix with a standard curve in neat solution, both spiked at equal concentration levels posterior to the sample preparation step (49,50). Slopes of the two curves can subsequently be compared by using a two-tailed t-test at a predefined significance level (50). However, a p-value does not provide any absolute numerical value that characterizes the matrix effect. For this reason, it seems more useful to calculate the matrix effect in the same way as in the post-extraction method, namely by calculating the percent ratio of the slopes of the standard addition curve and the standard curve in neat solution (4,16,49,51,52).
Limit of Quantification: In accordance with EMA and FDA guidelines on bioanalytical method validation, the limit of quantification (LOQ) is defined as “the lowest concentration of analyte in the sample which can be quantified reliably, with an acceptable accuracy and precision” (6,8). Acceptable refers to an accuracy value of 20% from the nominal concentration, and a precision value of less than 20%. Both guidelines further restrict the definition of LOQ as the lowest concentration level of the calibration curve that was created in authentic matrix. Another common approach to determine the LOQ is the determination of the concentration corresponding to a signal-to-noise ratio of 10 (53).
Both definitions impose some issues when the surrogate matrix approach is used. The EMA and FDA expect that the LOQ is evaluated in authentic matrix. When utilizing the surrogate analyte approach, the surrogate analyte can be used to determine the LOQ (30). Although the ICH draft guideline does not encompass any guidance concerning the determination of the LOQ when dealing with endogenous compounds, the standard addition method and the surrogate matrix approach demand an alternative approach.
In this context, it is interesting to discriminate between the instrumental LOQ (iLOQ) and the method LOQ (mLOQ) (54). While the iLOQ is a measure to characterize the performance of an instrument itself, the mLOQ considers the sensitivity of the actual method in the sample. The latter thereby considers potential loss in response as a result of, for example, procedural errors or matrix effects. The iLOQ can be easily determined in solvent. In contrast, the mLOQ is not as easily transferable to endogenously present molecules when SAM or the surrogate matrix approach is employed. There might be, for example, pronounced ion suppression present in the matrix, and as a result, the LOQ determined in solvent will differ from the LOQ in matrix.
For this reason, Tsikas suggested to define the LOQ as “a representation of the lowest added analyte concentration (CLOW+), which, upon addition to the biological sample, can be experimentally measured with acceptable accuracy (i.e. max. ± 20% deviation of the nominal value) and precision (max. RSD 20%)” (46). In the same way as for accuracy, the CM and C0, representing the concentrations upon spiking and the basal concentration, respectively, are determined by using either SAM or the surrogate matrix approach. The accuracy is calculated in accordance with the following formula, in which CLOW+ represents the lowest concentration of the spiked analyte that generates an accuracy value that falls within the limits (equation 9):
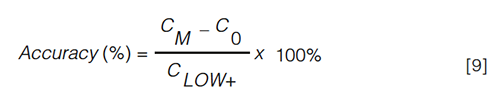
Conclusion
The quantification of endogenous compounds imposes a challenging task for the analyst because of the absence of a true blank matrix, which is required by FDA and EMA in the preparation of calibrators. Particularly in LC–MS, it is important to consider the influence of the matrix on the quantification as a result of matrix effects. Moreover, the current description of the validation parameters provided by the regulatory agencies provides a unique focus on the quantification of exogenous compounds and it should be appreciated that not all of them are directly transferable to the context of endogenous compounds. Very recently, the ICH has issued a new provisional guideline that contains guidance on the validation of methods used to quantify endogenous components (9).
A few ways are used to bridge the difficulties in the quantification process. Among them, the surrogate matrix approach offers the most straightforward way provided that an IS is available. Nonetheless, the mere availability of an IS does not guarantee instant success for accurate quantification, for example, in case of differences in elution time between the IS and the natural analyte. Its validity should therefore be thoughtfully addressed, not only in terms of coelution, but also in terms of mutual suppression. The surrogate analyte approach, on the other hand, offers the option to set the LOQ in the actual matrix without additional spiking and to prepare QC samples below the endogenous concentration levels, but its use might be limited due to the need for two ISs. In addition, in multicomponent analysis, utilizing an IS for each compound of interest might be impeded because of commercial unavailability. In the latter case, SAM might provide a viable alternative.
Regarding method validation, the use of the surrogate matrix or the surrogate analyte should be justified by proving parallelism between the surrogate matrix and the authentic matrix, or between the surrogate analyte and the authentic analyte, respectively. For this purpose, the comparison of slopes can be used as an alternative for the determination of accuracy at different concentration levels. As a result of the unavailability of a true blank matrix, the assessment of accuracy can be determined by comparing the difference between the concentration found after spiking and the endogenous concentration with the nominal spiked concentration. It is clear that in this way, accuracy cannot be determined below the endogenous concentration in the QC sample. In contrast, the surrogate analyte approach offers the advantage of evaluating accuracy below the endogenous concentration of the natural analyte. In the same way, the LOQ can be determined by simple use of the surrogate analyte rather than, as is the case for the other approaches, defining the lowest concentration that can be spiked to generate an accuracy value within predefined limits.
Coping with the unavailability of a blank matrix in this particular context might seem challenging at first sight. However, even without the use of an IS, quantification of endogenous concentration levels can still be achieved through the standard addition method. Of course, developing an adequate validation strategy is key in the generation of reliable results. The constant growth of the realm of metabolomics and biomarker research has prompted the regulatory agencies to provide a homogenous validation strategy regarding the quantification of endogenous compounds.
References
- J.K. Nicholson and J.C. Lindon, Nature455, 1054–1056 (2008).
- N.C. van de Merbel, TrAC - Trends Anal. Chem.27(10), 924–933 (2008).
- P.J. Taylor, Clinical Biochemistry38(4), 328–334 (2005).
- F. Gosetti, E. Mazzucco, D. Zampieri, and M.C. Gennaro, Journal of Chromatography A1217(25), 3929–37 (2010).
- A. Periat et al., J. Chromatogr. A1439, 42–53 (2016).
- US Food and Dug Administarion, Guidance for Industry: Analytical Procedures and Methods Validation for Drugs and Biologics (FDA, Rockville, Maryland, USA, 2015). https://www.fda.gov/media/87801/download
- National Institute of Health Sciences in Japan, Guideline on Bioanalytical Method Validation in Pharmaceutical Development (2013). Available at: http://www.nihs.go.jp/drug/BMV/250913_BMV-GL_E.pdf.
- EMA, Guideline on bioanalytical method validation (2012). Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf
- International Conference on Harmonization, ICH, Bioanalytical Method Validation M10 (Draft Version, 2019). Available at: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Multidisciplinary/M10/M10EWG_Step2_DraftGuideline_2019_0226.pdf
- R. Thakare, Y.S. Chhonker, N. Gautam, J.A. Alamoudi, and Y. Alnouti, Journal of Pharmaceutical and Biomedical Analysis128, 426–437 (2016).
- R. Thakare et al., Biomed. Chromatogr.32(3), (2018).
- D.A. Goncalves, B.T. Jones, and G.L. Donati, Microchem. J.124, 155–158 (2016).
- A.K. Hewavitharana, N.S. Abu Kassim, and P.N. Shaw, J. Chromatogr. A1553, 101–107 (2018).
- N.P. Shaw, S.K. Tan, and A.K. Hewavitharana, LCGC North Am.32(1), 54–64 (2014).
- SANTE/11813/2017, Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed. Eur. Comm. Heal. Consum. Prot. Dir. (2017). https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf
- E. Olesti, J. Rodríguez-Morató, A. Gomez-Gomez, J.G. Ramaekers, R. de La Torre, and O.J. Pozo, Talanta 192, 93–102 (2019).
- J. Chen et al., J. Pharm. Biomed. Anal.146, 361–368 (2017).
- J. Marcos and O.J. Pozo, Journal of Steroid Biochemistry and Molecular Biology162, 41–56 (2016).
- A.C. Suhr, M. Vogeser, and S.H. Grimm, J. Pharm. Biomed. Anal.124, 309–318 (2016).
- T. KamÄeva et al., J. Chromatogr. B Anal. Technol. Biomed. Life Sci.1001, 212–220 (2015).
- A.K. Hewavitharana, J. Chromatogr. A1218(2), 359–61 (2011).
- A. Van Eeckhaut, K. Lanckmans, S. Sarre, I. Smolders, and Y. Michotte, J. Chromatogr. B Anal. Technol. Biomed. Life Sci.877(23), 2198–207 (2009).
- S.S. Iyer, Z.P. Zhang, G.E. Kellogg, and H.T. Karnes, J. Chromatogr. Sci. 42(7), 383–7 (2004).
- S. Wang, M. Cyronak, and E. Yang, J. Pharm. Biomed. Anal. 43(2), 701–7 (2007).
- H.R. Liang, R.L. Foltz, M. Meng, and P. Bennett, Rapid Communications in Mass Spectrometry17(24) 2815–21 (2003).
- N.S. Abu Kassim, P.N. Shaw, and A.K. Hewavitharana, J. Chromatogr. A1533, 57–65 (2018).
- S. Ghassabian, L. Griffiths, and M.T. Smith, J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1000, 77–83 (2015).
- S. Kang, S.M. Oh, K.H. Chung, and S. Lee, J. Pharm. Biomed. Anal.98, 193–200 (2014).
- S. Ghassabian, N.S. Rethwan, L. Griffiths, and M.T. Smith, J. Chromatogr. B972, 14–21 (2014).
- S. Ongay et al., J. Chromatogr. A1326, 13–9 (2014).
- Y. Gong et al., J. Pharm. Biomed. Anal.111, 57–63 (2015).
- L. Liu et al., J. Chromatogr. B1011, 69–76 (2016).
- E. Rozet, R.D. Marini, E. Ziemons, B. Boulanger, and P. Hubert, Journal of Pharmaceutical and Biomedical Analysis55(4), 848–58 (2011).
- A. Kruve et al., Analytica Chimica Acta 870, 8–28 (2015). .
- O. González et al., Journal of Chromatography A1353, 10–27 (2014).
- C.P. Da Silva, E.S. Emídio, and M.R.R. De Marchi, Talanta131, 221–7 (2015).
- T. Singtoroj et al., J. Pharm. Biomed. Anal. 41(1), 219–27 (2006).
- A.M. Almeida, M.M. Castel-Branco, and A.C. Falcão, J. Chromatogr. B Anal. Technol. Biomed. Life Sci.774(2), 215–222 (2002).
- J. Van Loco, M. Elskens, C. Croux, and H. Beernaert, Accredit. Qual. Assur.7(7), 281–285 (2002).
- J.M. Andrade and M.P. Gómez-Carracedo, Anal. Methods5, 1145–1149 (2013).
- P. Steliopoulos, E. Stickel, H. Haas, and S. Kranz, Anal. Chim. Acta572(1), 121–4 (2006).
- X. Dong et al., Biomed. Chromatogr.33, 11 (2019).
- J. Martens-Lobenhoffer, S. Postel, U. Tröger, and S.M. Bode-Böger, J. Chromatogr. B Anal. Technol. Biomed. Life Sci.855(2), 271–5 (2007).
- R. Gurke et al., Talanta 204, 386–394 (2019).
- D. Tsikas, J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 877(23), 2244–51 (2009).
- D. Tsikas, J. Chromatogr. B Anal. Technol. Biomed. Life Sci.1093–1094, 80–81 (2018).
- R. Ottria, A. Ravelli, F. Gigli, and P. Ciuffreda, J. Chromatogr. B Anal. Technol. Biomed. Life Sci.958, 83–9 (2014).
- B.K. Matuszewski, M.L. Constanzer, and C.M. ChavezâEng, Anal. Chem.75(13), 3019–3030 (2003).
- B.D. van Zelst and R. de Jonge, J. Chromatogr. B903, 134–141 (2012).
- C.M. Teglia, M.D. Gil García, M.M. Galera, and H.C. Goicoechea, J. Chromatogr. A353, 40–8 (2014).
- B.K. Matuszewski, J. Chromatogr. B Anal. Technol. Biomed. Life Sci.830(2), 293–300 (2006).
- C. Steuer, P. Schütz, L. Bernasconi, and A.R. Huber, J. Chromatogr. B Anal. Technol. Biomed. Life Sci.1008, 206–211 (2016).
- European Pharmacopoeia (9th Edition) (2017).
- B. L’Homme, G. Scholl, G. Eppe, and J.F. Focant, J. Chromatogr. A 1376, 149–58 (2015).
Maxim Nelis is a Ph.D. student at the University of Leuven (KU Leuven) in Belgium. His current research focuses on the identification and quantification of small molecules in biological specimens collected from IBS patients with the objective to find a biomarker.
Patrick Augustijns is a professor in biopharmaceutics at KU Leuven. His laboratory has developed a unique technique to collect gastro-intestinal samples. Upon quantification of drugs and metabolites in these samples, this approach allows the gastroâintestinal behaviour of drugs and formulations to be linked to the systemic outcome.
Deirdre Cabooter is the editor of “Pharmaceutical Perspectives”. She is a research professor at the Department of Pharmaceutical and Pharmacological Sciences of KU Leuven. Her research interests include studying mass transfer in liquid chromatography, analyzing complex samples in diverse fields of application, retention modelling, and solutions for automated method development. She is also a member of LCGC Europe’s editorial advisory board. Direct correspondence about this column to the editorâinâchief, Alasdair Matheson, at amatheson@mmhgroup.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









