The State of the Art in Chiral Capillary Gas Chromatography
LCGC Europe
A review of the historical development and latest trends in phase development in the field of chiral capillary gas chromatography. A range of novel applications in chiral capillary GC are also described.
The present state-of-the-art in chiral capillary gas chromatography (GC) is reviewed in this article. The article provide a short historical overview of phase development followed by a comparison of GC and high performance liquid chromatography (HPLC) chiral separations. After a short discussion of mechanisms of the most popular cyclodextrin phases, practical recommendations on how to select the best stationary phase and then how to use it to optimize chiral GC separations are described. Examples of elution order reversal of racemates, injection of large volumes of diluted sample using retention gaps and other applications are also provided.
The origins of chiral gas chromatography (GC) technology can be found in an article by Volker Schurig and Leslie S. Ettre entitled "Emanuel Gil-Av and the Separation of Enantiomers on Chiral Stationary Phases by Chromatography" (1). For GC, there were two methods described, the direct approach utilizing chiral stationary phases with or without achiral derivatization and the indirect approach requiring derivatization of the racemate with a chiral reagent and separating the diastereoisomers on conventional achiral GC stationary phases. In today's world, the former is the preferred method given the extensive validation problems of chiral derivatization and the increased stability, reliability and diversity of available chiral stationary phases for capillary GC. In 1966, Gil-Av, Feibush and Sigler (2) achieved the first ever GC chiral separation using GC with N-trifluoroacetylLisoleucine lauryl ester chiral stationary phase (CSP) and separated N-trifluororacetyl alkyl esters of (±)-natural amino acids. Other stationary phases based on other amino acid or diamide derivatives have been developed and there are examples of chiral metal complexes that can interact with analytes through coordination complexation but none have been as successful or expansive as the cyclodextrin technology (3).

Chiral GC has seen a dramatic explosion of applications in many fields of study including natural products, asymmetric synthesis, biological studies, environmental contaminants, agriculture, food, flavour and fragrance with the evaluation of essential oils. See reference 3, in which 185 papers on chiral applications were reviewed using CSPs in capillary GC.
In this article, I attempt to review the types of columns that are currently available for chiral separations by GC, citing the advantages and disadvantages of each type. I also review the options available to solve a particular separation problem and the approaches to developing a separation. Optimization and troubleshooting tips are also included.
Advantages of GC Over HPLC
GC offers a number of advantages over high performance liquid chromatography (HPLC) for the separation of enantiomers including the ease of generating theoretical plate numbers ranging from 12000 to 60000, depending on column length, resulting in the separation of more pairs of enantiomers for each column; high speed of analysis meaning higher sample throughput; and with flame ionization detection (FID) as close to a universal detection method as you can get, so a lack of aromaticity or functionality of the target molecules are not negatives. The downside is that injected samples need to be quite clean of extraneous material (precolumns, so called retention gaps, can help here), have a vapour pressure below 250 °C (achiral derivatization can help here), and be thermally stable and not racemize (there are techniques that can help here such as lowering elution temperature with proper achiral derivatives). Please note that elution temperatures are related to analyte vapour pressure, not boiling point. There are also other advantages, such as short column equilibration time, ease of connection to mass spectrometry (MS) and ease of trace impurity quantitation.
Selectivity of other detection methods such as electron capture, the techniques of headspace extraction and the more routine use of two dimensional GC (GC×GC) have made the technique ideal for the analysis of enantiomers in complicated matrices like environmental, biological and agriculture samples. Development time and optimization are also faster than for HPLC. Increased thermal stability, high resolution, and increased peak capacity of current GC capillary columns make these tools ideal for the analysis of complex mixtures commonly encountered in samples from biological or natural sources. With current technological advances in CSPs there are often multiple choices for a particular racemate to maximize speed and increase efficiency leading to lower limits of detection. It is also easier to reverse elution order — that will be explained in more detail later. Also, despite the great success by the use of chiral LC, there are no chiral LC columns that can routinely separate a variety of volatile, nonaromatic enantiomers. And, of course, the biggest advantage is no waste solvent. The biggest drawbacks are the lack of preparative capabilities, the fact that the final analyte must be volatile and the inability to analyze thermally labile compounds.
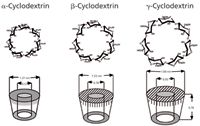
Figure 1
Competitive Chiral GC Technologies
Although HPLC has a wider variety of stationary phases (for example, π-complex, crown ethers, macrocyclic antibiotics and proteins, among others), cyclodextrin (CD) technology has been ubiquitous in the area of chiral stationary phases for capillary GC. CDs are cyclic, chiral, torus-shaped macromolecules composed of six or more D(+)-glucose residues bonded through α-(1–4) glycosidic linkage. The CDs containing six, seven and eight glucose units are the most common, best characterized and commercially available. There are now more than 16 different cyclodextrin chemistries on α-, β- or γ-cyclodextrin sold as chiral stationary phases for capillary GC (Figure 1). If you add in the differences in cyclodextrin that was derivatized, carrier type and concentration, bonded or unbonded that number increases to 45 choices. Unlike in HPLC, however, only a few of these columns serve the vast majority of required separations. There is one popular noncyclodextrin CSP that is widely used, Chirasil-Val, which is supplied by Agilent Technologies (Santa Clara, California, USA). This phase was based on the work of E. Bayer's group (4) and was introduced more than three decades ago. It was one of the earliest commercially available CSPs and is still most popular for the separation of D, L amino acids but has limited capabilities in other areas. It comes as either the L-valine or D-valine version. For the L-antipode, the D-amino acid is eluted first, which can be reversed using the D-valine CSP. For determining optical purity you can then choose the appropriate column so that the minor component elutes first, yielding the lowest possible detection limit. Using this column, a typical capillary GC separation of amino acids isolated by solid-phase extraction from green coffee beans that were derivatized as their N-ethoxycarbonylheptafluorobutyl esters is depicted in Figure 2 (5). As might be expected, roasted coffee beans showed almost no amino acids in their extract because they can't survive the roasting temperatures.

Figure 2
Features of CD Phases
The mechanisms of separation of CD technologies fall into two basic categories: surface interactions vs. inclusion. It is now known that certain CD derivatives are largely a surface interaction, being more efficient and with higher capacity while others involve the inclusion phenomena usually associated with cyclodextrin technology. Because the internal cavity of a cyclodextrin molecule tends to be more hydrophobic than its exterior, most nonpolar molecules (or molecules with nonpolar moieties) prefer to reside in the cyclodextrin cavity. This inclusion phenomenon in capillary chiral GC is controlled by the type and number of derivatives linked to the 3-position hydroxyl as opposed to the 2-position of the glucose units that make up the CD structure (see Figure 3). This article addresses these differences and how to use them as an advantage for a particular separation.
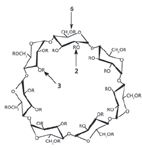
Figure 3
It should be noted that enantioselectivity is rare at temperatures above 200 °C. Temperature plays a significant role in chiral separations on GC — the lower the elution temperature the greater the selectivity differences. Thus, a compromise must be reached between retention time and selectivity enhancement in the separation of enantiomers.
Thermodynamic relationships and sample loading studies can be used to indicate the dominate chiral recognition mechanism. Enantiomers that have large H° and S° values also show low loading capacity. Sample capacity can be determined by injecting increasingly more sample on the column and monitoring the peak efficiency. As a rule of thumb, when the peak efficiency falls 10% from its underloaded value, the column is said to be overloaded. This suggests that inclusion plays a role in the enantioselective mechanism. Enantiomers that have smaller H° and S° values show a higher sample loading capacity. This implies that inclusion plays a less dominant, or negligible, role in the enantioselective mechanism. It is difficult to imagine that this is a black and white situation, but it is indicative of a situation that can prove advantageous. The second phenomenon that has been observed is the efficiency of peaks is much higher with those derivatives that indicate a surface mechanism as opposed to an inclusion mechanism. This is consistent with what has been observed with cyclodextrin derivatives in different solvent modes for HPLC.
The list of derivatives in Table 1 does not include column lengths or capillary diameters, only differences in chemistries and cyclodextrin type. Of the 45 choices in derivatives there are basically two chemical types, the alkylated version: methyl, ethyl, butyl or pentyl; and the polar type: acetyl, propionyl or the patented trifluoroacetyl. When more polar groups are placed in the 3-hydroxyl position of the glucose unit of the cyclodextrin the chiral interaction does not appear to involve the inclusion phenomena. These types of CSPs typically have higher efficiency, faster separations and capacities as high as 10–50 times greater than those relying on the inclusion phenomena. There is a second classification one can make in the choice between the coated versions and the derivatives bonded to polysiloxane. This bonding process increases the temperature stability only modestly, but there is a recognizable loss of selectivity in the bonding process (6). It is a consideration to be made based on the process needs, not in the method development scheme. Therefore, for screening purposes it is useful to have one version of each chemical type. The two this author recommends is the 2,3-di-o-methyl-6-tert-butyldimethylsilyl β-CD and the 2,6 dipentyl-3-trifluoroacetyl γ-CD. Further improvements in the method may come from the use of some of the other closely related derivatives.

Table 1: List of manufacturers with cyclodextrin-based columns for capillary GC.
Developing Protocols
There are two prime conditions for operating CSPs in GC. First, the recognition that the lower the analysis temperature, the greater the energy differences between the diastereomers; therefore, you want to operate at the lowest temperature possible. It is also possible to miss the window of separation by running at a temperature above the optimum elution temperature. Second, to achieve the best chiral selectivity in the shortest amount of time, it is advantageous to run at high linear velocities of carrier gas while using as low an elution temperature as possible. This is opposed to manipulating the temperature itself. Linear velocities of 100 cm/s and 60 cm/s are ideal for hydrogen and helium, respectively. To exploit high linear velocities when using temperature programming to find the window of separation, shallow ramp rates of 1–5 °C/min should be used if the elution temperature is below 130 °C and 5–10 °C/min if it is above 130 °C. It is important to use a good oxygen scavenger for all the CD phases especially when you exceed 110 °C. Oxygen, when present at the high temperatures used in GC, rapidly causes oxidation of stationary phases, resulting in column bleed, shortened lifetime and potential selectivity and retention changes.
Available CSPs for Chiral GC Separations
Every major supplier that has sold capillary GC columns in the past now has some version of a cyclodextrin CSP for GC. The leading CD derivative in the field from an application standpoint has been the 2, 3-di-o-methyl-6-o-tert-butyl dimethyl silyl β-CD. This has largely replaced the straight 2-3-6-tri-o-methyl version. The reason for this is the tert-butyl group allows for possible orientation of the CD in the polysiloxane carrier leading to greater selectivity and more efficient peaks. Comments in the literature indicating that the tert-butyl group blocks the entrance to the lower opening of the CD cavity has not been substantiated by experimentation. Here is a little background: the trimethyl β-CD derivative is a crystalline material and, as such, in a gas environment has poor mass transfer leading to poor peak efficiency and resulting in very broad peaks and high retention times. It has to be diluted with a polysiloxane carrier to make it more efficient to the tune of 20–30% CD to carrier. The tert-butyl group in the 6-position has little effect on chiral selectivity and therefore the site is derivatized to provide greater selectivity of the CD in the carrier. A number of different polysiloxane carriers are used, but no significant difference in selectivity has been noted from these changes. The isothermal operating temperature of this phase is usually the highest of all the CD phases at 220–230 °C, but when using a temperature ramp the upper temperature limit can be extended to 250 °C for short periods. This product has been shown to provide ~60% of all chiral separations possible on capillary GC phases. As in all of these types of cases, there are a few separations that still work better on the tri-o-methyl version, but it appears to be less than 10%. Furthermore, there are differences in the type, purity and concentration of carriers used by different manufacturers as well. As one would expect, as the elution temperature of the analyte gets lower the greater the influence of the carrier in retention of the analyte; so when working with low temperature analytes it is better to employ other types of derivatives that do not require a carrier. The differences from manufacturer to manufacturer for these phases are minimal for most separations. The main problem comes from those analytes that are eluted at low temperatures where the differences in concentration and purity of the carrier become significant. Table 2 lists classes of racemates suitable for the 2,3-di-o-methyl-6-TBDMS β-CD and the 2,6-di-o-pentyl-3-trifluoroacetyl γ-CD phases.

Table 2: Racemates suitable for separation by the two best chiral phases in GC.
For application breadth, the next CD derivative that could handle a good portion of the remaining 40% of the applications is the patented 2,6-di-o-pentyl-3-trifluoroacetyl γ-CD produced by Sigma-Aldrich/Supelco (Bellefonte, Pennsylvania, USA). The mechanism for this phase is a surface dipole–dipole interaction and as such covers a broader area using the γ-CD as opposed to the β-CD. This phase is highly selective for oxygen-containing analytes such as alcohols, ketones, acids, aldehydes, diols, epoxides, esters, phthalides, sulfoxides and lactones. This phase is also highly selective for halogenated compounds (alkanes) and amines. It separates not only the widest variety of enantiomers, but also the greatest number within certain classes. It has two limitations: its sensitivity to moisture and its isothermal upper temperature limit of 180 °C. This limit can be exceeded by 25 °C for short periods during temperature programming. The best defense to avoid hydrolysis is to use excess trifluoroacetic anhydride (TFAA) in your sample and to use a retention gap both after the injector and before the detector. A 5-m retention gap as a precolumn and 1-m to protect the column from the high temperature of the detector are recommended. Reference 7 demonstrates the application of this technique to monitor aqueous enzyme reactions. In a homologous series of alkane enantiomers, identical alpha values are observed regardless of chain length or branching indicating, only one or two carbons may be contributing to chiral recognition. Note that the sources of moisture that can effect this phase include moisture in the sample, carrier gas, or from air if not stored properly.
The versatility of Chiraldex G-TA can be seen in Figures 4 and 5. Separations of racemic mixtures of various classes of compounds are depicted in these figures.

Figure 4
When a carbonyl is present from a trifluoroacetyl, acetyl or propionyl group bonded to the 3-position and the 2-position is either a methyl, ethyl or pentyl group, the chiral interaction of analyte favours the surface interaction mechanism of the CD as opposed to cavity inclusion mode. Separations are typically faster and more efficient. The added advantage is the capacities are generally higher for this mechanism as opposed to those derivatives that promote an inclusion interaction. The trifluoroacetyl is the more effective of the three possible derivatives based on a review of the applications followed by acetyl then propionyl. The inclusion mechanism recognizes analyte size, shape and functionality and, therefore, is useful for certain target analytes. The other derivatives that are available offer incremental improvements over the types already stated.
Figure 5
Approaches to Obtaining Chiral Selectivity
The easiest approach in selecting a CSP is to take advantage of the many free laboratory services offered by column manufacturers. Turnaround time is usually short in comparison to HPLC and you are typically guaranteed to get the best column available for the job at hand. Many of the manufacturers will also consult with you on the phone to give you some direction for column selection and method development. The second, more time-consuming, approach is to search the many databases available on the web or from the manufacturers. If your molecule of interest is new and not closely related to what has been reported then search the list of class categories typically supplied by the manufacturers. For example, the dipentyl derivatives of α-, β-, and γ-CD demonstrate selectivity differences based on size, shape and functionality. α-CD is useful for simple epoxides, cyclic ethers and linear substituted amines; β-CD is useful for heterocyclic amines; and γ-CD is useful for the naphthyl analogs. The derivatives are typically listed with that kind of description. However, you now have 25 possible choices to study. Also there is the extensive literature that is available in the various fields where this technology has been used. A number of application reviews can help here (3,8). Finally, you can take my recommendations of the two most widely applied CD columns and run temperature ramps as recommended above.
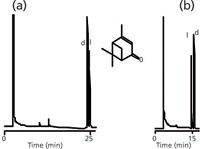
Figure 6
Reversal of Elution Order
There are three basic methods that have been used with this chiral column stationary phase technology to reverse elution order. The most effective is to switch the size of the cyclodextrin, for example β to γ or vice versa (see Type I in Figure 6). The second method is to change the mechanism, going from a surface interaction phase like 3-trifluoroacetyl-2-6-di-o-pentyl γ-CD to an inclusion mechanism phase like 2,6-di-o-pentyl-3-butyl γ-CD (see Type II in Figure 7). Finally, but least effective, is to change the derivatizing agent, for example, from trifluoroacetyl to acetyl for an amine chiral site (Type III in Figure 8). Be aware that this approach can also change selectivity so it is not always appropriate. Reversal of elution order methods becomes valuable when you are developing a trace analysis separation and want to have the impurity peak eluted first so that the column can be loaded with sample and the impurity will not interfere with the smaller trace peak. If the smaller impurity peak appears on the tail of a very large peak, there is a chance that it will appear to be unresolved or not be seen. This can also result in the integrator not defining an accurate peak area or retention time and poor quantitation results. It has also been useful here to use a retention gap of up to 5 m to load the sample onto the separation column. Here, a slow injection of a large volume of sample (up to 5 µL) is performed at a temperature below the elution temperature of the sample, but above the vapour pressure of the solvent.
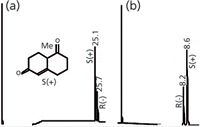
Figure 7
Examples of three basic methods (Types I–III) for reversal of racemate elution order can be seen in Figures 6, 7 and 8. An example of the use of a 5-m retention gap to load a diluted sample onto a GC chiral column is shown in Figure 9. The use of the retention gap prevents localized overloading of the stationary phase and allows the analytes to be widely separated from the large ethanol solvent peak.
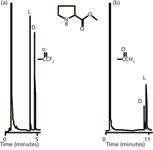
Figure 8
Optimization
There are a number of influences on chiral selectivity in this technology. Achiral derivatization of the racemate influences chiral selectivity. It is therefore necessary to investigate several types of derivatives to determine ultimate success. Usually, the larger the analyte, the smaller derivative size is most effective. For small molecules the reverse may be true. In addition, elution order can be affected by the type of derivative chosen and can be used to affect elution of the trace enantiomer first to maximize sensitivity. See Table 3 for a list of successful derivatizing agents and preferences.
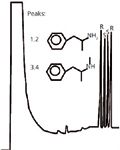
Figure 9
It is suggested that work at the lowest possible elution temperature and using an increased carrier gas flow will reduce retention time. Usually a 30-m column is considered the workhorse but a 10-m scout column can be a handy, quick guide to find the required window of separation. Remember that both moisture and oxygen can affect selectivity as well as the column and analyte stability. Therefore, it is necessary to use good drying and oxygen traps for continued optimal performance.

Table 3: Achiral derivatization methods.
Three carrier gases have been used in this technology: nitrogen, hydrogen and helium. Nitrogen offers low cost and high efficiency. The disadvantage of nitrogen is the optimal linear velocity is 12 cm/s and efficiency is rapidly lost with increasing linear velocity. Helium is more expensive, but the optimum linear velocity is 20 cm/s and the slope of HETP vs. linear velocity is flatter. Hydrogen has an optimum linear velocity of 40 cm/s, has the flattest HETP vs. linear velocity of all and the lowest viscosity, but workers often question its safety. Helium, therefore, is a good compromise. As stated above, differences between diastereometric complexes need to be established first through the use of low elution temperatures; the use of hydrogen will result in more efficient peaks. There are cases where highly volatile racemates may be investigated and in those cases nitrogen would be the preferred carrier gas.
Conclusions
In this instalment, I have discussed the current status of the separation of enantiomeric compounds by GC. All in all, the CD phases have achieved the greatest success, and, unlike HPLC, only a few phases are required to cover the gamut of volatile, derivatized or nonderivatized enantiomeric compounds. I have discussed retention mechanisms and provided recommendations for column selection as well as suggested operating conditions. Some application examples of the use of chiral stationary phases were shown including their versatility, ability to reverse racemate elution order, the separation of derivatized amino acids in green coffee beans and the use of retention gaps to inject large volumes of diluted chiral compounds.
This article was originally published in "Column Watch" in the August 2011 issue of LCGC North America.
Thomas E. Beesley is the founder and former CEO of Quantum Industries, manufacturer of thin-layer chromatography (TLC). He designed preadsorbent and channeled TLC and sold to Whatman 1978. He is also the founder and CEO of Advanced Separation Technologies Inc. (ASTEC), which manufactured chiral HPLC, Cyclobond, Chirobiotic lines and chiral GC Chiraldex phases. That company was sold to Sigma-Aldrich in 2006. Tom is currently a contract problem solver, expert witness and lecturer as a chiral specialist for Chiral View LLC.
"Column Watch" editor Ronald E. Majors is a senior research scientist at the Columns and Supplies Division, Agilent Technologies, Wilmington, Delaware, USA, and is a member of LCGC Europe's editorial advisory board. Direct correspondence about this column should go to "Column Watch", LCGC Europe, 4A Bridgegate Pavilion, Chester Business Park, Wrexham Road, Chester, CH4 9QH, UK or e-mail the editor, Alasdair Matheson, at amatheson@advanstar.com.
References
(1) V. Schurig and L.S. Ettre, LCGC N. Amer., 25(4), 382–395 (2007).
(2) E. Gil-Av, B. Feibush and R. Sigler, Tetrahedron Lett., 7(10),
1009–1015 (1966).
(3) L. He and T.E. Beesley, J. Liq. Chromatog. & Related Tech., 28, 1075–1114 (2005).
(4) H. Frank, G.J. Nicholson and E. Bayer, J. Chromatogr. Sci., 15, 147 (1974).
(5) S. Casal, M.R. Alves, E. Mendes, M. Beatriz, P.P. Oliveira and M.A. Ferreira, J. Agric. Food Chem., 51, 6495–6501 (2003).
(6) Y. Tang, Y. Zhou and D.W. Armstrong, J. Chromatogr. A, 666(1–2), 147–159 (1994).
(7) L. Duchateau, S. Wildeman, W. Koolen and J. Mommers, "Chiral GC Analysis of Aqueous Samples by On-Column Derivatization: Application for Enzyme Reactions," Chirality Busan, Korea, June 2006.
(8) V. Schurig, J. Chromatog., 906, 275–299 (2001).
Errrata
In addition to the companies mentioned MEGA DEX chiral columns are also available in the marketplace.
The 26th Norwegian Symposium on Chromatography
March 29th 2024The 26th Norwegian Symposium on Chromatography was held 21–23 January 2024. The symposium has strong traditions in the Norwegian separation science community, serving as a forum for excellent scientific talks, networking, and social events.






