Nomenclature and Conventions in Comprehensive Multidimensional Chromatography – An Update
LCGC Europe
A review of nomenclature and conventions used in comprehensive multidimensional chromatography. An update of the popular paper on this topic from 2003.
Comprehensive two-dimensional chromatography necessitates additional nomenclature over and above that used in classical one dimensional (1D) chromatography. The use of two (or more) columns requires various parameters on these separate dimensions to be adequately identified. This is achieved by use of a superscript preceding the respective symbol. Also, the process of coupling different dimensions leads to specific terminology, which we report here. A number of recent examples where specific nomenclature related to retention indices, estimation of first dimension retention times, and the definition of modulation ratio, are included. This article and its predecessor aims to establish a basis for suitable nomenclature and conventions in this rapidly advancing area.
In 2003 Schoenmakers et al. published a position paper in LC•GC Europe (1) defining various nomenclature and conventions in comprehensive multidimensional chromatography. This paper was the outcome of a session organized to discuss the terminology and nomenclature used in this emerging field, during the First International Symposium on Comprehensive Multidimensional Gas Chromatography (GC×GC), held on 6–7 March in Volendam, the Netherlands.
This first symposium was the progenitor to eight further symposia so far, with the 9th GC×GC scheduled for Riva del Garda, Italy, in May 2012. Many of the listed conventions and nomenclature were based on practices that had appeared in the literature up to that time – and admittedly this was largely in the field of GC×GC. Perhaps not surprisingly, unanimous agreement was reached on many of these definitions, terms and symbols, and the need to ensure that establishing a basic ‘set of conventions’ was appreciated. Most of the terms were logical and this aided acceptance and understanding among chromatographers.
At that time, there were relatively few reports on LC×LC or other comprehensively coupled separation methods. Since this first meeting, many LC×LC developments have been introduced, with a number of different implementation formats, and the review of Stoll et al. in 2007 should be referred to (2). In addition, the 2003 paper included some proposals for nomenclature and conventions that were recommended even though they had not been used in the literature. According to Scopus, that paper has been cited almost 90 times in subsequent literature (becoming the most highly cited paper in LC•GC Europe), and so has been accorded good acceptance within the chromatography community.
It is time to update the earlier review with various terms and conventions that have been described since that time in this rapidly advancing field of chromatographic analysis. In addition, comments on methods for derivation of various parameters for comprehensive multidimensional chromatography experiments will be included. The more extensive literature on LC×LC in recent years has resulted in a range of new terminologies being added to the lexicon of comprehensive twoâdimensional chromatography and this justifies the addition of terms peculiar to that experimental arrangement.
For completeness, the Tables of the earlier review will be reproduced here and additions included where appropriate.
There still remains some lingering confusion or inconsistency over the use of the words “comprehensive” and “orthogonal,” and of the multiplex sign (×) as a short notation for comprehensive two-dimensional (2D) separations. Hence, as in the 2003 paper, the background to, and further comment on, these aspects will be included.
Comprehensiveness
An important discussion surrounded the argument of when a 2D separation can be called comprehensive. Three criteria were established, based essentially on the definitions formulated earlier by Giddings (3). There is a need to clearly distinguish between classical (heart-cut) 2D separations and comprehensive 2D separations. Also, some practitioners use the term ‘comprehensive GC analysis’ to mean ‘analysing everything’.
However, when a specific part of the sample is removed prior to (by selective sample preparation) or during the analysis (for example, solvent vent or a split-flow arrangement), it is still possible to refer to the separation as comprehensive. In that instance it is good practice to specify the fraction of the sample that is analysed. For example, a comprehensive 2D separation of a specific plant extract will refer to theselected extract, which acknowledges the inherent limitations of extraction selectivity (not all the components may be extracted equally). In such an instance the sample can be redefined as that well-defined fraction of the initial material to be analysed. Thus the extract (and not the plant) is the sample to be analysed by comprehensive 2D chromatography. Similarly, the sample can be a serum obtained from whole blood, a distillation fraction obtained from crude oil, or a specific fraction obtained from a chromatographic separation, etc. Therefore, the guiding principle (3) is that a 2D separation can be called comprehensive if:
- Every part of the sample is subjected to two different (independent) separations;
- Equal percentages (either 100% or lower) of all sample components pass through both columns and eventually reach the detector; and
- The separation (resolution) obtained in the first dimension is essentially maintained.
The third criterion can never be met completely, but chromatographers have learned to live with arbitrary distinctions in similar contexts (for example, the extra-column contribution to peak broadening must be kept negligible). Clearly Giddings understood that criterion 3 was a key to differentiate the heart-cut process from what he envisioned as the proper comprehensive separation experiment, but he will not have conceived how the column chromatography experiment could be conducted to ensure the 1D separation was fully maintained. Blumberg and Klee (4) have recently revisited this definition and re-cast it as “An n-dimensional (nD) analysis is one that generates n-dimensional displacement information”.
One way that the qualifier “essentially maintained” (point 3) may be translated into practical terms is to specify that a reduction in the apparent 1D resolution (observed by projecting a specific peak pair in the comprehensive 2D chromatogram on the 1D axis) should not exceed some fixed limit (for example, 10%). However, this has not been paid due attention in the literature. The most important aspect of preserving resolution in the first dimension is the number of 2D chromatograms recorded across the width of a 1D peak, with three or four normally referred to as the minimum target number. It should be noted that the desire to “essentially maintain” the separation achieved in the firstâdimension may not coincide with the optimum conditions of analysis. For example, Horie et al. (5) and Vivó Truyols et al. (6) independently came to the conclusion that around two “cuts” per 1D peak is optimal for achieving efficient LC×LC separations.
In the earlier paper, the second criterion was reported to give rise to much discussion. Most delegates felt that the term “comprehensive” should not be reserved for instances in which every analyte is completely (100%) transferred to the 2D column and then recorded at the detector - provided that the “split factor” x (%) is equal for all components. If (apart from a single intensity factor of x) the obtained chromatogram is identical to the one obtained with 100% transfer, then the term comprehensive may be used. In simple words, a “faithful representation” of the sample must be obtained in truly comprehensive 2D chromatography. In practice, it is not easy to obtain such a faithful representation if the transfer factor is not 100%. By selecting time fractions for transfer to the 2D column (for example, sending the effluent to waste during 90% of the time), the fractions of individual peaks will vary. This may result in the comprehensive 2D chromatography experiment being a qualitative process, rather than a quantitative method. When flow splitters are used, gas chromatographers hardly need to be reminded of the danger of discrimination such devices entail. If in doubt (i.e., when some discrimination may occur), it is better to speak of nearâcomprehensive 2D separations.
The sub-sampling interfaces (i.e. those that sample less than 100% of the injected material and can sample or modulate each solute to a different amount) include diaphragm and early (differential) flow modulators in GC, and instances where the volume transferred to, for example, dual sampling loops in LC exceed the break-through volume of the loop.
A final point worth making is that “comprehensive gas (or liquid) chromatography” is not the same as “comprehensive two-dimensional gas (or liquid) chromatography.” The former is an imprecise usage of language that should be avoided. Shorter, unambiguous abbreviations are discussed below. Likewise, it is noted that sometimes the literature includes terms such as “comprehensive GC×GC chromatography”. This is also an imprecise use and should be avoided.
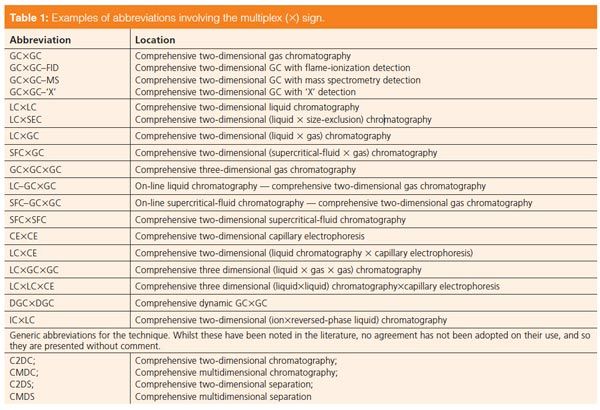
Table 1: Examples of abbreviations involving the multiplex (×) sign. [Click to Enlarge]
The Multiplex Sign
To abbreviate the term “comprehensive 2D” the multiplex sign (×) may be used (7). This is a clear distinction from conventional “linear” or “heartâcut” 2D separations, which are usually abbreviated with a hyphen (or n-dash) (for example, GC–GC, LC–GC). The multiplex sign can be used as an abbreviation for a number of different comprehensive 2D separation techniques. Some examples are listed in Table 1. Hyphens are conventionally used to indicate the coupling of a chromatographic system to any kind of detector: as in GC with flame-ionization detection (GC–FID), GC coupled with Fourier-transform infrared spectroscopy (GC–FTIR), or GC with atomic-emission detection (GC–AED), although usage such as GC/MS is often noted), and to indicate on-line coupled (“hyphenated” or “coupled-column”) systems (such as on-line solid-phase extraction in combination with LC, SPE–LC). This illustrates that hyphens are used in several different ways and that the meaning of the symbol is not unambiguously defined. However, in all three instances the hyphen indicates that the systems are coupled on-line.
Other variants to coupled instrument notations include GC(FID) for “simple” (single-channel) detectors and LC/MS or LC//MS for off-line combinations, and even variations within abbreviations for GC with time-of-flight mass spectrometry, such as GC–TOF/MS, GC–TOF–MS or GC–TOF MS for this instrumental arrangement are noted. A detailed discussion of such notations and obtaining some consensus in their use is beyond the scope of the present paper. For a discussion on the notation of chromatography–MS systems see the glossary of terms proposed by Murray (8).
Because MS is a technique to separate ions generated on-line with the separation step, one can argue that GC×MS and LC×MS are comprehensive 2D separation methods. This argument is most realistic when a soft ionization method is used, which results in minimal fragmentation of parent ions. This has been used, for example, for GC×FIMS (field ionization MS), or other similarly soft ionization methods, with data displayed on largely independent axes of retention and ion mass (which predominantly generate molecular ions).
Every analyte component is then indeed separated in two dimensions. If MS acquisition takes place sufficiently fast to meet the requirements of comprehensiveness and if discrimination at the MS inlet is avoided, the ‘×’ sign may arguably be used. For conventional gas chromatography–mass spectrometry with electron ionization, a hyphen is more appropriate. The abbreviation GC×2GC is suggested for a comprehensive two-dimensional gas chromatography system with two parallel second-dimension (2D) columns (9). According to the guidelines given above for use of the word “comprehensive,” this is applicable to such a system, provided that the sample is distributed across the two 2D columns without any discrimination. An example of a comprehensive three dimensional separation (GC×GC×GC) has been noted (10).
A point that may have been noted by authors in recent years is the lack of consistency in the expression used for the abbreviation GC×GC. Of 15 journals checked, eleven used GC×GC and four used GC × GC. Other infrequent and inaccurate uses include GCXGC, and GCxGC (that is, using the normal font as either capital or lower case, instead of the multiplex sign). There appears to be consensus that GC×GC without spaces should be used.
Table 2: Definitions of orthogonality in different fields of science. [Click to Enlarge].

Orthogonality
“Orthogonality” remains one of the terms used most inconsistently in the literature on 2D separations. This is quite unlike the precise definitions in mathematics and statistics. In the chemical literature, orthogonality concepts are often associated with multidimensional instrumental chemical analysis and especially with separation science, where it has been used informally to indicate merely different separations (coupled separation dimensions) or mechanisms (hyphenated with detection), rather than those that are “perpendicular” or “independent.”
In the Volendam discussion, no reasons were found for such a broad and peculiar interpretation of the word. A sensible definition for “orthogonality” in separation science is listed together with definitions from mathematics and statistics in Table 2. The analytical definition implies that completely independent analysis processes are used, or in separation science that independent retention mechanisms apply in the two dimensions.
Whether or not orthogonal separation mechanisms (orthogonal separations) lead to good separations depends on the sample, its components and the specific physical-chemical manner in which the retention mechanisms are exploited. For example, if 1D separates according to boiling point, while 2D is only able to separate according to stereo-selectivity, then only stereoâisomers in the sample will exhibit an orthogonal 2D separation. Sometimes it is noted that authors refer to the ‘degree of orthogonality’ in a separation. For instance, LC×LC comprising different LC mechanisms – such as, reversed-phase and normal phase; size-exclusion and reversedâphase should meet this criterion. Note in contrast with the GC×GC experiment both dimensions have an underlying analyte boiling point mechanism. The presumed ‘orthogonal’ column set comprising a non-polar 1D stationary phase with a polar 2D stationary phase has led some authors to refer to a polar×non-polar stationary phase column set as non-orthogonal. There should be no justification for this designation. There is still scope for a rational basis and agreement for determining the ‘extent of orthogonality’.
In the following section, some recent studies that support certain concepts in GC×GC are further exemplified. The following sections refer to the nomenclature and terms reported in Table 3.
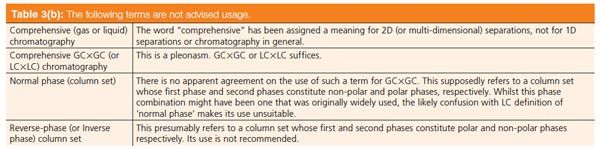
Table 3(a): Nomenclature suggested for comprehensive two-dimensional (gas) chromatography. [Click to Enlarge]
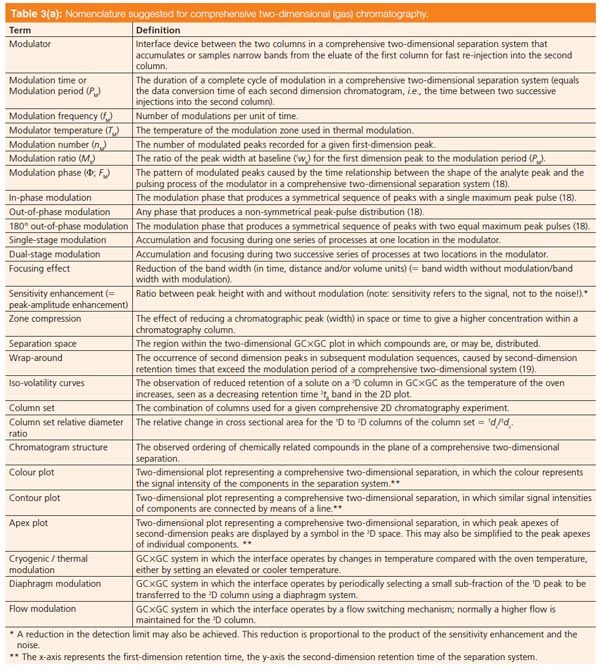
Table 3(b): The following terms are not advised usage. [Click to Enlarge].
Retention Indices, II values have been reported for 1D and 2D data in GC×GC, and the experiment can be conducted in a number of ways. 1D data are best acquired by using a ‘split T’ before the 2D column, with 1D retention data (1tR) recorded with an FID. However, such an arrangement must lead to an incorrect assessment of the actual 1tR value due to added retention on the short transfer line that connects the 1D column to the FID. Others have used the retention time of the largest modulated peak (or the modulation time of this peak) in the GC×GC result as a surrogate for the 1tR value, and then used this for deducing the 1I values (11).
A method for 2I in GC was reported by using a suite of alkanes injected periodically through the first injector, which requires them to pass through the entire first column (12). The interference caused by co-injected solvent was a drawback to the method. A solid-phase micro-extraction (SPME) method with appropriate alkane sampling was then described (9), with again injection into the first injector. The constraint of passing alkanes through the 1D column was overcome by delivering alkanes directly to the end of the 1D column just prior to the modulator, so that any alkane could be introduced to the 2D column at any time. The use of a second injector made this possible, with SPME again used for sampling the alkanes so that no solvent was introduced.
In all cases, the sequentially injected alkanes describe a so-called ‘isoâvolatility’ plot within the 2D separation space, which refers to the observation that the injected component defines an exponential reduction in 2tR as temperature increases. Interpolation (or sometimes extrapolation) between successive alkanes in the pseudoâisothermal 2D analysis gives the 2I value. The method is not straightforward and demands some attention to the details of the experiment. However, one can predict that once the 2D space has been defined by suitable alkane elution patterns to derive an equivalent 2I space, the 2I value of any solute should be easily deduced.
Apparent 1tR Values from Modulated 2tR Peaks
The modulated peaks that are generated from the GC×GC experiment can be used to estimate the apparent 1tR value on the 1D column. We choose to call this 1tR,app because it is a derived value rather than a directly measured quantity. A model describing the resulting distribution of 2D peaks, based on a 1D Gaussian peak shape, can be fitted to the 1D peak (13, 14). The prediction of 1tR,app also has to include measurement of the solute hold time in the modulator, which we choose to call th, and must also account for the 2tR value.
Note that the th value is not simply the same as the modulation time or modulation period (PM; in some literature, this has been proposed as the second dimension cycle time 2tc). The total retention time of a modulated peak in the experiment should take into account all retention contributions to the total. It might be thought that a simple formula such as:
tR,tot = 1tR + 2tR + th [1]
will account for the different contributions to the total. However, an accurate 1tR value cannot be easily defined for a single modulated peak – due to the manner in which the first peak is sampled in the experiment. There is no specific 1tR value for the segment or part of the 1D peak that is sampled, but rather a range of retention times for the segment of the sampled peak. However, 1tR values can be estimated by applying a Gaussian or exponentially modified Gaussian (EMG) function to the distribution of modulated peaks for the component, and deriving the peak maximum for this reconstructed 1D peak.
The Modulation Ratio; MR
The modulation ratio was introduced (15) as a means to quantify the relationship between PM, to the peak width at baseline on the first column (1wb). In many cases the time available for the 2D separation is equal to PM. In some cases (for example, when using gradient-elution LC for 2D in the LC×LC experiment) the available time may be shorter. The obvious question to ask is how does one decide upon the appropriate PM to use? Note here that in LC, the time available to the 2D separation has been termed 2tc, the second dimension cycle time. LC normally does not involve a focusing ‘modulation’ step (nor a timeâcompression effect as in flow modulation in GC), so modulation period PM might not be interchangeable with 2tc for LC×LC.
The modulation ratio (MR) is defined as 41σ / PM, where 41σ is the measure of 1wb. The modulation ratio is approximately equal to the number of second-dimension chromatograms (“cuts” or “slices”) recorded for each 1D peak. For a given 1D peak, the modulation time will be shorter than the peak width at base if MR > 1. MR can easily be established, since both PM and 1wb are or should be known and this can aid in method set-up (16). The number of modulated peaks recorded (the modulation number, nM) depends on the magnitude (injected mass) of the component, the modulation phase, the modulation period relative to the peak width and the sensitivity of detection of the 2D peaks for the compound. Thus, nM cannot be rigorously defined.
A number of experimental parameters can be set based on the desired MR value and the value of PM derived from the 1wb value. For instance, PM determines the maximum value of 2tR that can be recorded without wrap-around. Under nonâprogrammed conditions 2tR is related to 2k and 2tM (or the equivalent term 2t0 in size-exclusion chromatography).
Other Definitions
In Table 3 a number of other relevant definitions are collected. Several of these are specifically derived from the field of GC×GC, but their use need not be exclusive for this technique. For example, the word modulator may equally well be used to describe the intermediate stage in a number of other comprehensive multidimensional chromatography methods. One result from the Volendam discussion was that a modulator does not necessarily need to incorporate a focusing effect; in fact there is no rationale for insisting that focusing is necessary according to the definitions of comprehensive multidimensional chromatography. However, GC×GC is most readily accomplished in practice with the aid of focusing. The latter is easily achieved by thermal modulation in GC×GC, but the increasingly popular method of flow modulation does not rely on a focusing effect in space. However, it does have a compression effect in time. Focusing is also not usually encountered in the comprehensive 2D combination of two liquid-phase separation methods.
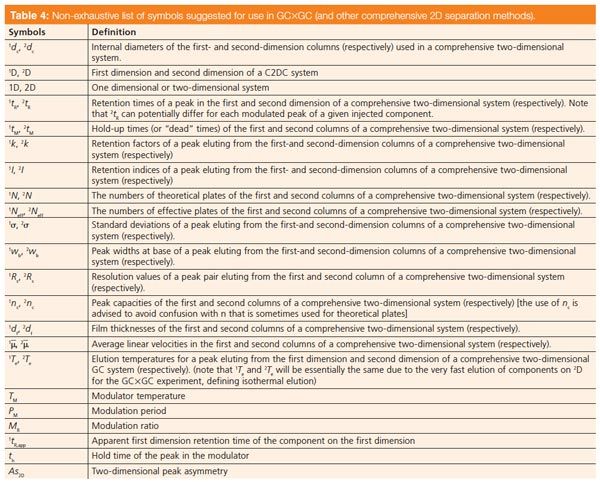
Table 4: Non-exhaustive list of symbols suggested for use in GC×GC (and other comprehensive 2D separation methods). [Click to Enlarge].
Symbols
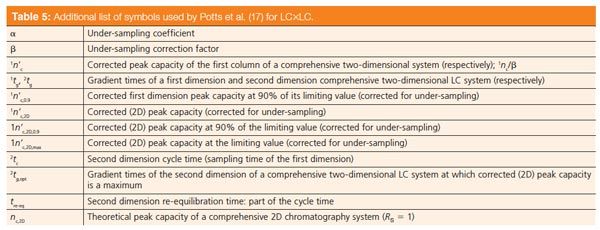
Table 4 lists a number of symbols that are recommended for use in comprehensive 2D separations in general and GC×GC in particular. The use of the superscript prefix 1 or 2 is suggested to distinguish between the 1D and 2D columns. This table has a number of more recent examples of symbols that have been reported for GC×GC. Table 5 reports a selection of nomenclature proposed for aspects of LC×LC according to a recent paper (17), in which the authors state that they endeavour as much as possible to base their nomenclature on that used in the 2003 LC•GC Europe article.
Table 5: Additional list of symbols used by Potts et al. (17) for LC×LC. [Click to Enlarge].
Conclusion
Comprehensive 2D separations are increasingly important for the separation of complex mixtures. In this paper, a number of suggestions for definitions, nomenclature and symbols are presented that originated from the community involved with comprehensive 2D gas chromatography (GC×GC), but may be equally applicable for other comprehensive 2D separations. Some examples of studies that illustrate determination of specific parameters in the GC×GC experiment are outlined.
The use of clear, unambiguous and well-defined terms and symbols may assist in better mutual communication between separation scientists, and between separation scientists and the broader scientific community.
References
- P.J. Schoenmakers, P.J. Marriott, J. Beens, LC•GC Europe, 16(6), 335–339 (2003).
- D.R. Stoll, X. Li, X. Wang, P.W. Carr, S.E.G. Porter, S.C. Rutan, J. Chromatogr. A, 1168(1), 3–43 (2007).
- J.C. Giddings, J. High Resol. Chromat.,10(5), 319–323 (1987).
- L. Blumberg, M.S. Klee, J Chromatogr. A, 1217(1), 99–103 (2010).
- K. Horie, H. Kimura, T. Ikegami, A. Iwatsuka, N. Saad, O. Fiehn, N. Tanaka, Anal. Chem., 79(10), 3764–3770 (2007).
- G. Vivo-Truyols, S. van der Wal, P.J. Schoenmakers, Anal. Chem.,82(20), 8525–8536 (2010).
- J.C. Giddings, Anal. Chem., 56(12), 1258A–1270A (1984).
- K.K. Murray, J. Chromatogr. A,1217(25), 3922–3928 (2010).
- S. Bieri, P.J. Marriott, Anal. Chem., 78(23), 8089–8097 (2006).
- N.E. Watson, W.C. Siegler, J.C. Hoggard, R.E. Synovec, Anal. Chem.,79(21),
- 8270–8280 (2007).
- Z.L. Cardeal, P.P. de Souza, M. da Silva, P.J. Marriott, Talanta, 74(4), 793–799 (2008).
- R.J. Western, P.J. Marriott, J. Chromatogr. A,1019(1–2), 3–14 (2003).
- L.L. Xie, P.J. Marriott, M. Adams, Anal. Chim. Acta, 500(1–2), 211–222 (2003).
- J.L. Adcock, M. Adams, B.S. Mitrevski, P.J. Marriott, Anal. Chem., 81(16), 6797–6804 (2009).
- W. Khummueng, J. Harynuk, P.J. Marriott, Anal. Chem., 78(13), 4578–4587 (2006).
- W. Khummueng, P.J. Marriott, LC•GC Europe,22(1), 38–45 (2009).
- L.W. Potts, D.R. Stoll, X. Li, P.W. Carr, J. Chromatogr. A,1217(36), 5700–5709 (2010).
- R.C.Y. Ong, P.J. Marriott, J. Chromatogr. Sci.,40(5), 276–291 (2002).
- P.J. Marriott, R.M. Kinghorn, R. Ong, P. Morrison, P. Haglund, M. Harju, J. High Resolut. Chromatogr., 23(3), 253–258 (2000).
Ze-ying Wu is currently a Ph.D. candidate in the School of Chemistry at Monash University, Australia.
Peter Schoenmakers is a staff member in the van’t Hoff Institute of Molecular Sciences, Faculty of Science, University of Amsterdam, the Netherlands.
Philip Marriott joined the School of Chemistry, Monash University, Australia, in 2010, from RMIT University, Australia.
“Coupling Matters” editor Robert Shellie has extensive experience in hyphenated techniques and is currently Associate Professor at Australian Centre for Research on Separation Science (ACROSS), University of Tasmania, Hobart, Australia and a member of LC•GC Europe’s editorial board. Direct correspondence about this column should go to LC•GC Europe editor, Alasdair Matheson, 4A Bridgegate Pavilion, Chester Business Park, Wrexham Road, Chester, CH4 9QH, UK or e-mail amatheson@advanstar.com.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









