Sorptive Tape Extraction — A Novel Sampling Method for the in vivo Study of Skin
LCGC Europe
An important prerequisite of a good sampling procedure for skin sebum is its reproducibility.
A sorbent tape based on polydimethylsiloxane is an excellent method for the in vivo sampling of sebum at the surface of human skin. The technique is called sorptive tape extraction (STE). Enrichment of sebum by STE has been compared with data obtained with a Sebumeter and the reproducibility of STE sampling is discussed. Qualitative and quantitative analysis of the sebum sampled is performed by thermodesorption on-line with gas chromatography–mass spectrometry (TDS/GC–MS) or by liquid desorption followed by high temperature GC–MS (LD/HTGC/MS). The STE method permits the study of all the important sebum components, including free fatty acids, squalene, cholesterol, waxes and triglycerides. The performance of the method is illustrated with the determination of squalene, which is known to be one of the main sebum components responsible for the skin's shininess, and the effect of a matifying product on the skin's shininess. Other applications of STE are briefly presented.
Improving the knowledge of skin and evaluating the efficacy of cosmetic products more effectively are two fundamental objectives of R&D in the cosmetic industry. These objectives can be reached by characterizing the skin composition and its modifications by environmental factors. The purpose of our approach was to determine qualitatively and quantitatively the chemical composition of the skin surface and of the upper layers of the stratum corneum as they are representative of skin conditions and can be studied in vivo and in a non-invasive way. More precisely, the research focused on the investigation of sebum at the skin surface in vivo.
A variety of techniques have been described to assess sebum in vivo. The pioneering method is a gravimetric analysis of sebum adsorbed on cigarette paper.1 Other methods are based on adsorption of sebum on an opalescent material2 or on a glass surface,3 followed by photometric analysis. These techniques have some merits but suffer from poor reproducible sampling and/or provide only a global quantitative figure. More in-depth skin analysis was obtained through direct collection of the sebum at the skin surface by solvent rinsing4 or by extraction of the sebum sampled on an adsorbing material, such as Sebutape,5 followed by thin layer chromatography analysis (TLC). The first method, rinsing the skin with an organic solvent, presents an ethical problem while the latter method requires a time-consuming sample preparation step.
In recent years, different solventless extraction techniques have been introduced. Best known are solid-phase microextraction (SPME)6 and stir bar sorptive extraction (SBSE).7 In SPME and SBSE using polydimethylsiloxane (PDMS) enrichment is governed by the well-known chromatographic partitioning mechanism. Unfortunately, both techniques cannot be used for skin applications as the contact surface is too small. Therefore, for planar sampling on the skin, a tape composed of PDMS was developed. The tape is a flexible PDMS sheet (typically 15 mm×4 mm for thermodesorption and 15 mm×12 mm for liquid desorption) with a film thickness of 0.5 mm. The design is perfectly adapted for sampling at skin surface because tapes are flexible, giving good contact with the skin. Additionally, they are non-occlusive and sampling is ambulatory, easy and fast, which means large panels of volunteers can be studied. Moreover, compared with adsorptive enrichment, this mode of trapping and enrichment is highly reproducible, predictable and displacement effects do not occur as the mechanism is equilibrium controlled.
Solutes enriched on the inert PDMS material can be desorbed by temperature or by a liquid. Thermal desorption (TD) on-line coupled to GC–MS is applied for the routine analysis of free fatty acids and squalene while liquid desorption (LD) followed by high temperature GC–MS is the method of choice to unravel the complexity of waxes and triglycerides, and to detect cholesterol and its derivatives. LD is less sensitive compared with TD by the solvent dilution effect.
Sampling and Analytical Procedures
Collection of sebum samples is illustrated in Figure 1. The PDMS tape is placed on the skin by means of a plaster. After sampling, the PDMS tape can easily be removed for thermal or liquid desorption.
Figure 1: Volunteer with two PDMS tapes on two symmetric surfaces of the face (hemi-foreheads). Note that each area is divided in three sub-areas for sampling in function of time.
An important prerequisite of a good sampling procedure for skin sebum is its reproducibility. Total quantities of sebum sampled for 15 min on 15 mm×4 mm sorbent tape were determined by weighing on an ultra-microbalance UMX2, sensitive to 0.1 μg (Mettler Toledo, Greifensee, Switzerland). On the members of a panel of four volunteers with normal or greasy skin, two sorbent tapes were placed on two symmetric sites in the centre of the two hemi-foreheads (left and right sides). Sebum samples were collected during 5 days, at 9 am and 3 pm for each volunteer. This selection (timing, skin types etc.) is arbitrary and has no influence on the results as only the reproducibility on the average of each left/right individual data (n=40) was evaluated. The data, expressed as μg of sebum sampled/cm2 of sorbent tape are summarized in Table 1 showing highly reproducible values.
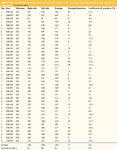
Table 1: Sebum quantities sampled by STE on left and right sides on four volunteers, twice a day during 5 days (μg of sebum sampled/cm2 of sorbent tape).
The coefficients of variation between left and right sides were calculated for each individual data set. The average coefficient of variation (n=40) is 8.8% ± 8.6%. This coefficient of variation is small considering the fact that the sebum is collected on a living human being. Values of the coefficients of variation are much higher for the conventional techniques (i.e., 15.6% for the cigarette paper method,1 14.6% for the solvent extraction technique4 and 16.2% for the Sebutape method5 ). The reproducibility of these results also confirms that a sampling time of 15 min is close to equilibrium for the skin target solutes (see further) between these two media.
Data with the new sampling method were also compared with the lipidic index obtained using a Sebumeter SM 815 (Courage & Khazaka, Koln, Germany). The sebum adsorbed on an opalescent film modifies its transparency that is measured by photometry. Results are expressed as μg sebum/cm2 of skin. The correlation was studied by comparing the quantities of sebum sampled by sorbent tapes on the left side and by the Sebumeter on the right side of the vertical median line of the body. The study was achieved on 10 volunteers, on four skin areas (forehead, cheek, back and chest) during 2 days (n=80). The correlation between the two techniques of R=0.9 (R2 =0.8) shows good linearity and, thus correlation (Figure 2).
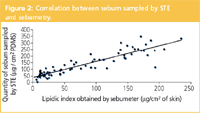
Figure 2: Correlation between sebum sampled by STE and sebumetry.
Note, however, that the two techniques do not measure exactly the same. The Sebumeter is a tool classically used to estimate the sebum casual level that means the quantity of sebum present at the skin surface at a given time. The STE method, as described here, gives a sampled sebum quantity related to the casual level. The sampled quantity indeed is controlled by the partition coefficient of sebum components between the skin and the sorbent material (partition coefficients are not discussed in this article). However, the sebum sampled by STE is also in relation with the sebum excreted during the sampling time; but it is known that the sebum excreted during a 15 min sampling time is small in comparison with the casual level, especially when no degreasing was done before sampling. STE has also been applied to assess specifically the sebum excreted rate after skin degreasing before the sampling. This application is not discussed in this article.
For detailed qualitative and quantitative chemical characterization of the sebum constituents, two complementary analytical methods have been developed. In STE method 1, a PDMS tape of 15 mm×4 mm with a thickness of 0.5 mm is placed on the skin for 15 min. The PDMS sheet is then introduced in a thermal desorption tube for TDS/GC–MS analysis. In STE method 2, the sampling procedure is the same but the sheet is 15 mm×12 mm×0.5 mm df. The sheet is extracted by immersion in 1 mL acetone during 20 min under agitation. A 1 to 5 μl extract is analysed by high temperature (HT) GC–MS.
The instrumental set-up for thermal desorption consists of a TDS-2 thermodesorption unit (Gerstel, Mülheim an der Ruhr, Germany) mounted on a 6890N/5973N GC–MS (Agilent Technologies, Little Falls, Delaware, USA). Desorption is performed at 300 °C during 20 min under a helium flow of 50 mL/min. Desorbed analytes are focused in a PTV injector CIS-4 (Gerstel) at -100 °C prior to transfer onto the analytical column at a split ratio of 1:20.
The separation is performed on a free fatty acid phase (FFAP) column (25 mL×0.25 mm i.d.×0.20 μm df) CP-WAX 58 (Varian, Middelburg, The Netherlands) at a constant flow of 1 mL/min. The GC oven is programmed from 80 °C at 10 °C/min to 180 °C and then at 3 °C/min to 250 °C (20 min). MS acquisition was in electron impact scan mode with range 35–500 m/z. High temperature GC–MS of the liquid extracts was performed on a 6890/5973 GC–MS combination equipped with a cool-on-column injector. The analytical column 10 mL×0.32 mm i.d.×0.10 μm df HP1 column was connected to a retention gap 4 mL×0.25 mm i.d. (Agilent Technologies). The carrier gas is helium at constant pressure (21 KPa). The GC oven was programmed from 50 °C (2 min) at 10 °C/min to 360 °C (5 min). MS acquisition was from 50 to 800 m/z.
Qualitative and Quantitative Study of Sebum
Sebum is produced by the sebaceous glands, epidermal appendixes located in the dermis. In the glands, the sebum is mainly composed of squalene (ca. 15%), wax esters (ca. 25%) and triglycerides (ca. 60%) with some traces of cholesterol and its derivatives. During the move from the glands to the skin surface, some of the triglycerides are converted into free fatty acids by the resident microflora. Sebaceous lipids are very complex and more than 200 different fatty acids have been identified. Nicolaides, a pioneer in cutaneous lipid analysis, has stressed the biochemical uniqueness of human sebum.8
Figure 3 shows a typical analysis using STE method 1 in combination with TDS/GC–MS. The main component is squalene, "the" most important tracer of sebum as it only occurs in such high quantities in human skin (with the exception of shark liver). The other peaks are covering the saturated and mono-unsaturated fatty acids in the C12 to C19 range originating from the bacterial decomposition of the triglycerides. The identity of the acids can be elucidated by GC–MS and the absolute quantities measured. Most important features of sampling method 1 in combination with thermal desorption are its simplicity (no sample preparation required) and high sensitivity. Moreover, the sample is collected on a very narrow skin surface of 0.6 cm2 . This is particularly interesting in the cosmetic field to study several skin areas of the face of one person (for example, for kinetic studies or for comparison of the efficacy of several cosmetic formulas applied at the same time). Other lipidic solutes such as α-hydroxy acids can also be studied by the described methodology (data not shown). In-situ hydroxyl derivatization reactions for sorptive extraction methods have recently been described.9,10
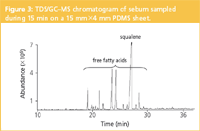
Figure 3: TDS/GCâMS chromatogram of sebum sampled during 15 min on a 15 mmÃ4 mm PDMS sheet.
Liquid desorption of the larger tape allows in-depth qualitative and quantitative analysis of all lipids of sebum. Figure 4 shows the analysis of the acetone extract of a 15 mm×12 mm×0.5 mm df tape. The sebum profile contains the free fatty acids, squalene, cholesterol, wax esters, a cholesterol ester and the triglycerides. The structure of more than 100 compounds could be elucidated. Some typical mass spectra, representing each class of compounds are shown in Figure 5(a)–(e). The complexity of skin sebum is further illustrated in Figure 5(f) showing the extracted ion chromatogram at m/z 368, ion typical for cholesterol esters. More than 10 cholesterol esters could be identified. We have to note, however, that in the original chromatogram silicone oligomers originating from the sheet dominate the profile. The lipid fraction could easily be elucidated by ion extraction at m/z 57; an ion that occurs in all lipids.
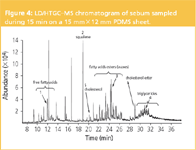
Figure 4: LD/HTGCâMS chromatogram of sebum sampled during 15 min on a 15 mmÃ12 mm PDMS sheet.
While the first method only allows partial analysis of the sebum components, the second method requires a larger sampling area (1.8 cm2 ) and is less sensitive but enables the simultaneous analysis of all lipidic classes present in sebum. The fact that an off-line prepared liquid sample is obtained by method 2 opens other perspectives, such as detailed analysis of a target solute by concentration of the sample and/or large volume injection and by derivatization of specific functionalities, such as hydroxyl and aldehyde groups.
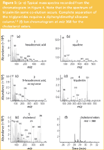
Figure 5: (aâe) Typical mass spectra recorded from the chromatogram in Figure 4. Note that in the spectrum of tripalmitin some co-elution occurs. Complete separation of the triglycerides requires a diphenyldimethyl siloxane column.12 (f) Ion chromatogram at m/z 368 for the cholesterol esters.
Skin care applications
STE has a broad applicability in the cosmetic field, particularly for skin care products. The two main applications identified are
- the improvement of skin knowledge by studying specific markers that allow the skin state to be characterized
- following the efficacy of applied cosmetics and studying the bioavailability of the ingredients.
The first application embraces the study of numerous tracers, such as sebum, epidermal, sweat, inflammation, oxidative stress etc. The evolution of these tracers, according to environmental factors, can also be investigated, for example, the qualitative and quantitative evolution of sebum at the skin surface in relation to an acute psychological stress. In the second application (i.e., the efficacy of cosmetic products), numerous effects can be studied, for example, matifying, seboregulating, anti-perspirant, deodorant, hydrating, anti-ageing, anti-oxidant, soothing, anti-pollutant properties, as well as the cutaneous penetration or non-penetration of actives.
By way of illustration, the action of a matifying product on the sebum present at the skin surface using STE method 1 is presented. The squalene level is monitored as this compound is known to be one of the main sebum components responsible for the shiny skin. The effect of a matifying product was studied on a panel of 25 volunteers over a period of 4 h. On two symmetric surfaces of the face (hemi-foreheads) one area was used as reference (no product application) while on the other area the matifying product was applied. Each area was divided in three sub-areas) for sampling at t0 (before product application) and, at 30 min and 4 h after product application (Figure 1).
Figure 6(a) shows the quantity versus time plot for squalene. The results, average levels of the 25 volunteers, are presented in Table 2. The plot shows that there is no significant difference at t0 between the two hemi-foreheads and that the matifying effect of the product starts immediately after its application (–66% of squalene quantity after 30 min compared with the non treated area) and keeps its activity after 4 h (–33% of squalene quantity). Figure 6(b) shows the results for the same experiment but obtained using a Sebumeter SM 815 (Courage & Khazaka, Koln, Germany) at t0 before the product application, 1 h and 4 h after the product application. These results don't prove the matifying effect of the studied product as no decrease of the lipidic index on the treated area compared with the untreated area was observed. To the contrary, the lipidic index on the treated area increased drastically after the product application, while the index is relatively stable on the untreated area, confirming the inaccuracy of the results obtained by the Sebumeter in this instance.
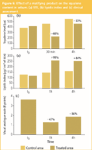
Figure 6: Effect of a matifying product on the squalene content in sebum. (a) STE, (b) lipidic index and (c) clinical assessment.
The excellent performance of STE for this study was proven by comparison with the clinical assessment of the skin shininess. Figure 6(c) presents the average clinical scores given by the dermatologist (using a six points-visual analogue scale from 0 matt to 5 very shiny) at t0 before the product application and, 1 h (47% of the shininess score compared to initial values) and 4 h (36% of the shininess score compared to initial values) after the product application. The Sebumeter, based on a pressure-sensitive adsorption mechanism, appeared not at all appropriate for the demonstration of the matifying effect of the studied product. Conversely, the STE method appears to be a good alternative to classical instrumental tools.

Table 2: Comparison of the squalene levels (ua) on control and treated areas using STE method over a 4 h period. Average data (n=25).
Other applications
The applications of STE in the cosmetic field seem endless, for example, the study of perfumes, toiletries and make-up products on skin. The STE technique can also be applied in numerous other fields dealing with the elucidation (qualitatively and quantitatively) of organic substances on surfaces.11 These include aroma compounds on flowers and fruits; contaminants, such as pesticides and endocrine-disrupting chemicals on indoor furniture, walls and floors; street drugs and pharmaceuticals; organic contaminants; and analysing the migration of bisphenol A from polycarbonate bottles.
Conclusion
Sorptive extraction (SE) on PDMS is known to be an excellent solventless extraction technique. Developing tapes to increase the contact area for solid surfaces has broadened the SE applicability. The potential of STE has been illustrated with a cosmetic application related with the assessment of skin shininess. A study on the efficacy of a matifying cosmetic product could be conducted, which was hitherto impossible. The performances of STE (reproducibility, sensitivity, etc.) offer great opportunities for the in vivo investigation of tracers related to skin ageing and skin hydration state to mention but a few, on skin.
Acknowledgement
We thank Sandra Casamassima and Fabienne Venturoli for technical assistance.
Sandra Sisalli is manager of the Skin Applied Analysis Laboratory at the Cosmetic Research Center, Chanel Parfums et Beauté, located in Sophia Antipolis, France. Adrien Adao is head of the Analytical Department at the Cosmetic Research Center, Chanel Parfums et Beauté, located in Sophia Antipolis, France. Manhattan Lebel is project leader in the Cosmetic Efficacy Evaluation Department, Chanel Parfums et Beauté, located in Paris, France. Isabelle Le Fur is head of the Cosmetic Efficacy Evaluation Department, Chanel Parfums et Beauté, located in Paris, France. Pat Sandra is director of the Research Institute for Chromatography, Kortrijk, Belgium and Professor in Organic Chemistry at the Ghent University, Ghent, Belgium.
References
1. J.S. Strauss and P.E. Pochi, J. Invest. Dermatol., 36, 293–298 (1961).
2. G.E. Pierard et al., Skin Pharmacol. Appl. Skin Physiol., 13, 372–389 (2000).
3. D. St Leger, Arch. Dermatol. Res., 265, 79–89 (1979).
4. M.R. Ruggieri et al. in "Advances in Thin Layer Chromatography, Clinical and Environmental Applications", Ed. J.C. Touchstone, Publ. John Wiley & Sons, New York, USA, pp 249–259 (1982).
5. K.M. Nordstrom, J. Invest. Dermatol., 87(2), 260–263 (1986).
6. C.L. Arthur and J. Pawliszyn, Anal. Chem., 62, 2145–2148 (1990).
7. E. Baltussen et al., J. Microcolumn Sep., 11, 737–747 (1999).
8. N. Nicolaides, Science, 186, 19–26 (1974).
9. M. Kawaguchi et al., J. Chromatogr. A, 1041, 19–26 (2004).
10. A. Stopforth et al., J. Chromatogr. B, 2005 (submitted).
11. P. Sandra, unpublished results.
12. E. Geeraert and P. Sandra, J. High Resolut. Chromatogr., 8, 415–422 (1985).

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









