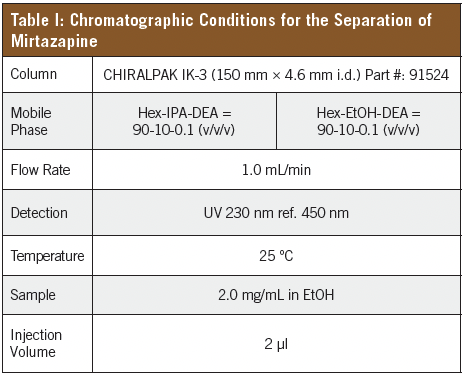
Separation of Mirtazapine on CHIRALPAK® IK-3
Mirtazapine is an approved antidepressant, with other studies exploring its use to treat sleep apnea, substance abuse withdrawal symptoms, and as an antihistamine. The (S) enantiomer, esmirtazapine, was under investigation as a treatment for insomnia and vasomotor symptoms associated with menopause. For these reasons, it would be helpful to establish an effective chiral analysis allowing for rapid and accurate quantification.
Although there are already several methods published in the literature for the separation of mirtazapine, they all present some less-than-ideal characteristics, whether it be a large particle size, unnecessarily long analysis time, or poor resolution.
Daicel’s newest immobilized chiral stationary phase (CSP), CHIRALPAK IK-3, has been found to provide a better alternative to these published methods. CHIRALPAK IK-3 is an immobilized cellulose-based CSP functionalized with tris (3-chloro-5-methylphenyl) carbamate selectors, and offers unique separations characteristics not available in any other commercially available chiral phase. The added robustness coming from the immobilization process increases its utility as a result of expanded solvent compatibility.
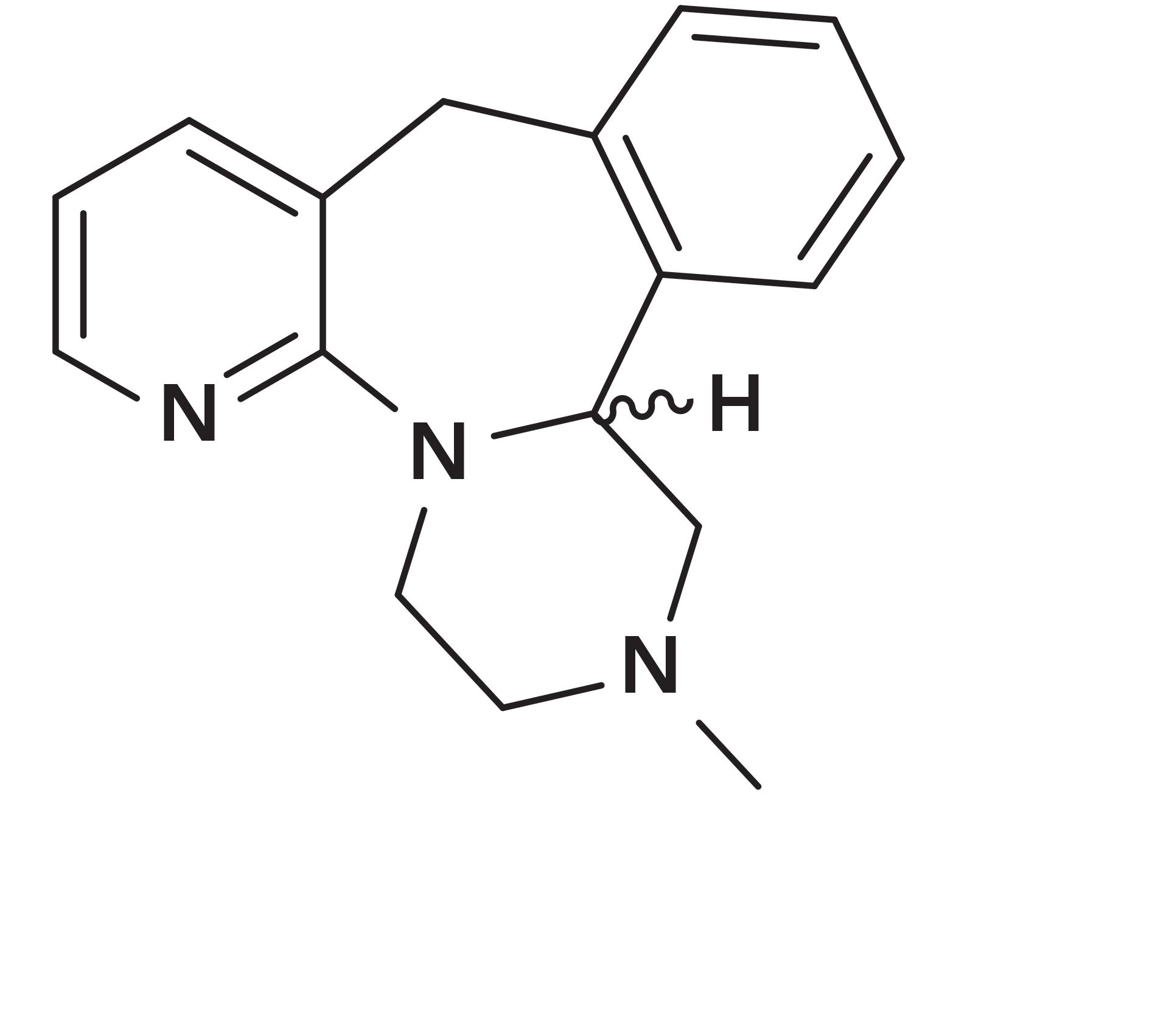
Figure 1: mirtazapine
Experimental
Mirtazapine and diethylamine (DEA) were purchased from Sigma Aldrich, and used as is. The solvents used were all purchased from Pharmco, were HPLC-grade or higher, and were used as is. Specifically, the hexanes (Hex) contained 95% n-hexane. The ethanol (EtOH) was reagent alcohol, which contains 90% ethanol, 5% methanol, and 5% isopropanol (v/v/v). Initial screening and optimization were performed on an Agilent 1200 equipped with a quaternary mixing pump and utilized a DAD.
Discussion
Mirtazapine was prepared as a 2.0 mg/mL solution in ethanol, and screened on Daicel’s library of 3 µm immobilized CSPs with Hex-EtOH-DEA = 80-20-0.1 (v/v/v) and Hex-IPA-DEA = 70-30-0.1 (v/v/v). This produced several partial or near-baseline separations. Of those separations, CHIRALPAK IK-3 exhibited more favorable characteristics of a chiral separation, that being good peak shape and selectivity with a reasonable retention time. Other columns showed longer retention and/or a greater degree of tailing. The separations on IK-3 were optimized by increasing the retention by decreasing the percentage of alcohol. For Hex-IPA, it was found that 10% isopropyl alcohol yielded a baseline separation of the enantiomers, with an analysis time of less than 6 min.
Figure 2: Separation of mirtazapine on CHIRALPAK IK-3 with Hex-IPA
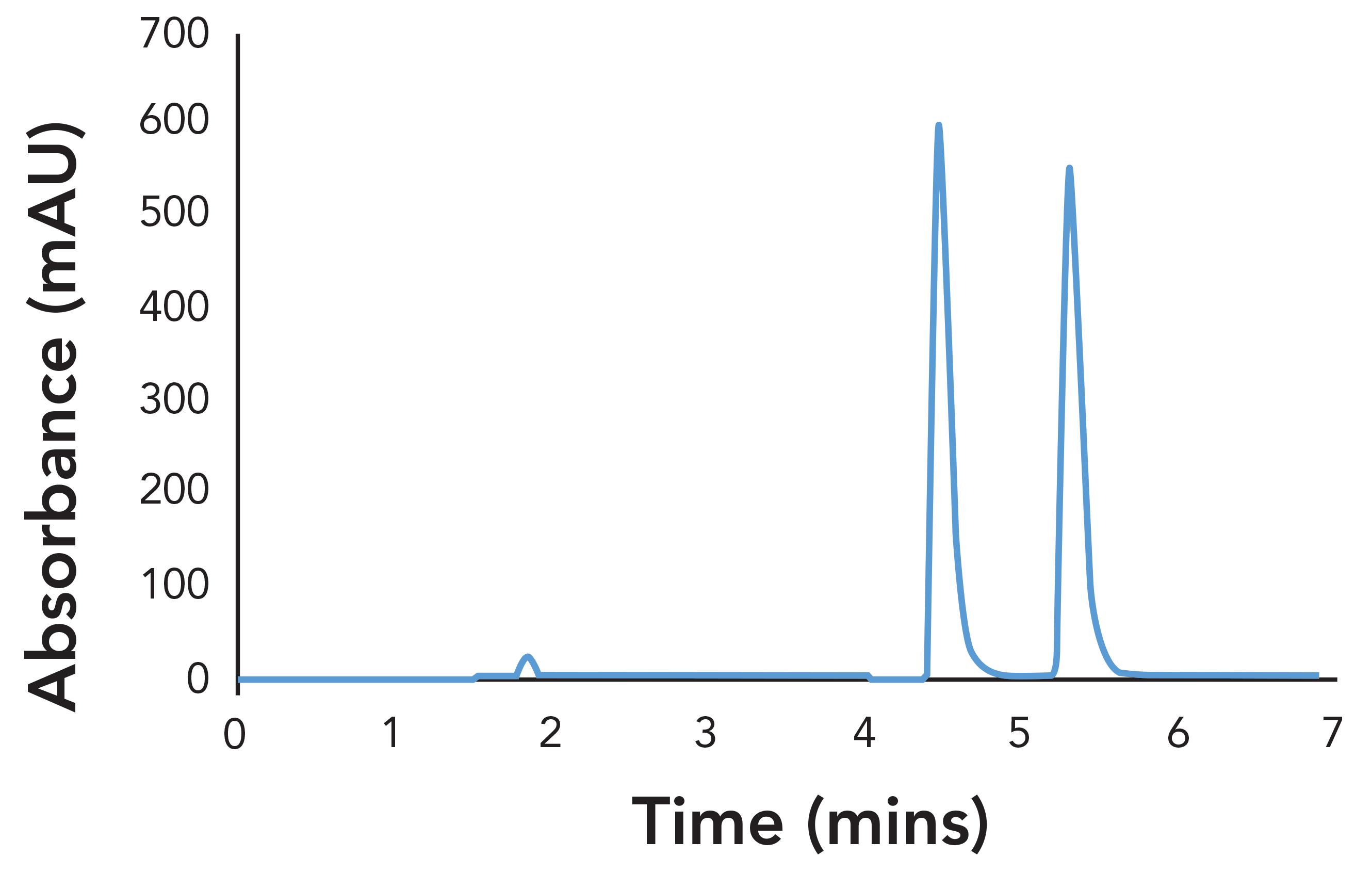
Figure 3: Separation of mirtazapine on CHIRALPAK IK-3 with Hex-EtOH
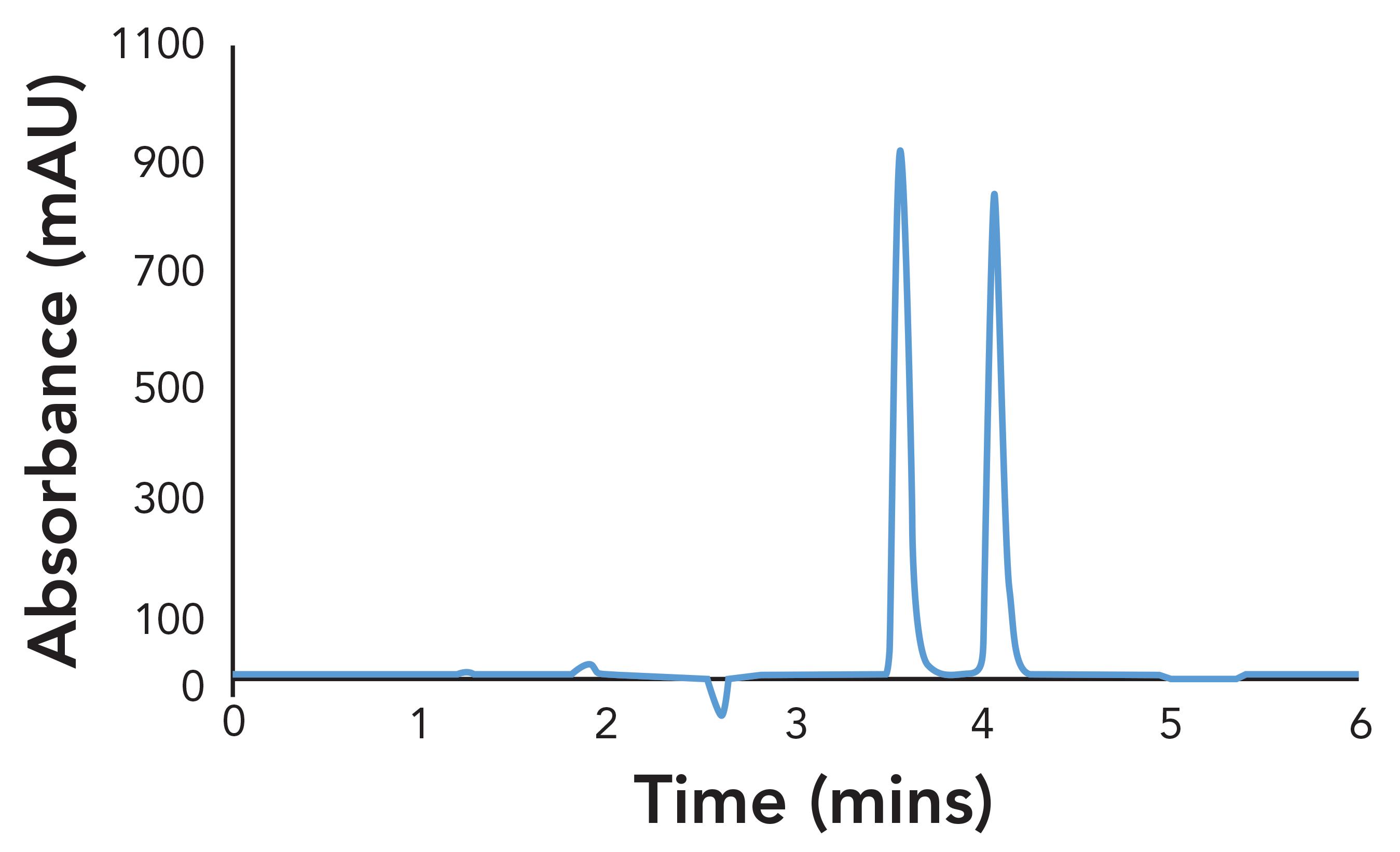
For Hex-EtOH, 10% alcohol also produced a baseline separation in under 4.5 min, with good peak shape and selectivity. Typically, a smaller percentage of ethanol is required to produce a similar separation to IPA, as it is a stronger eluting solvent. However, in this case, ethanol helps improve the peak shape (less tailing), and thus 10% can be used to further reduce the analysis time.
Conclusions
The unique separation characteristics of CHIRALPAK IK-3 yielded new baseline separations for mirtazapine not previously reported in the literature. These should serve as important methods for the analysis of mirtazapine and esmirtazapine going forward. It also shows that continued development of new chiral stationary phases is important. The best way to affect selectivity and separation performance is by changing the phase system (solvents or CSP). With mobile phase combinations well established, the greatest potential for finding new separations is with the release of new chiral selectors.

Daicel Chiral Technologies
1475 Dunwoody Drive, Suite 310 West Chester, PA 19380
Tel. 800-6CHIRAL
Website: chiraltech.com
The 26th Norwegian Symposium on Chromatography
March 29th 2024The 26th Norwegian Symposium on Chromatography was held 21–23 January 2024. The symposium has strong traditions in the Norwegian separation science community, serving as a forum for excellent scientific talks, networking, and social events.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)

