Selectivity in Reversed-Phase LC Separations, Part I: Solvent-Type Selectivity
LCGC North America
Methanol and acetonitrile are the two most popular organic solvents to use in the mobile phase for reversed-phase liquid chromatography (LC) separations. Tetrahydrofuran is also used to a lesser extent. Why are these solvents the most popular ones? Are there alternative solvents that should be tried? This month's "LC Troubleshooting" examines these and some other aspects of solvent selection for LC.
In the 1970s, Rohrschneider made an extensive study of the properties of various solvents (1). Among other things, he showed that, in some cases, when two solvents were mixed, properties of a third solvent could be obtained. This suggested that some subset of all possible solvents could be used for chemical purposes. Around the same time, Lloyd Snyder was interested in the various solvent properties that were important in liquid chromatography (LC) separations, especially reversed-phase separations (2,3). From other studies, he knew that three properties were of particular significance in terms of selectivity: the acidic, basic, and dipolar nature of the solvents. Snyder recalculated Rohrschneider's data based on these three properties and plotted them in what is now known as the solvent selectivity triangle. A subset of these data is shown in Figure 1, where each solvent is plotted according to its proportion of acidic, basic, and dipolar properties. The heavy dots show the central tendency of several solvent classes, some of which I have labeled.

John W. Dolan
In principle, three solvents — one with 100% acidity, one with 100% basicity, and one with 100% dipolarity — would be located at the extreme corners of the triangle. If these solvents existed, we should be able to blend them in different proportions and get the properties of solvents lying within the boundries of the triangle. Of course, such solvents do not exist. Most solvents have a mixture of acidic, basic, and dipolar properties, so they lie away from the corner and usually away from the edges of the triangle. Since the desired solvents don't exist at the corners of Figure 1, we might consider other solvents that are close to the corners — solvents that exhibited primarily acidic, basic, or dipolar properties. For example, carboxylic acids (RCOOH in Figure 1) are quite acidic, amines (for example triethylamine) are basic, and the chlorinated solvents (for example, dichloromethane, CH2Cl2) have dipole properties. If we were to blend these three solvents together, we might expect to get intermediate properties. But again, we are foiled, because the chlorinated solvent doesn't mix with the others and two phases result — not a desirable mobile phase characteristic! We can continue working our way toward the center of the triangle, searching for solvents that are miscible and have a dominance of one of the desired properties. For example, alcohols (such as methanol) have some acidic properties, ethers (for example, tetrahydrofuran) have basic properties, and nitriles (for example, acetonitrile) have dipole properties. These three solvents are mutually miscible and form a subset (shaded area) of the total triangle. It would be nice to conclude that because of Snyder's work, these three solvents were selected as the solvents of choice for reversed-phase separations, but this would be a bit presumptuous. By the time this work was completed, these three solvents were already the most popular solvents, but now we know why they had been found to be most effective. The enhanced acidic, basic, and dipolar properties of one solvent can assist certain chemical interactions better than another mobile phase solvent, so that difficult-to-separate solutes can now be separated.
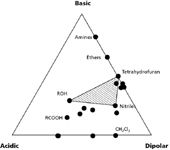
Figure 1: Classification of solvent properties according to their acidic, basic, and dipolar nature. Adapted from references 2 and 3.
Practical Applications
One popular application of the solvent selectivity triangle is based on a set of seven experiments using various solvent blends. This strategy formed the basis of the Sentinal system introduced by DuPont when they were in the LC business and was adapted as the Perkin-Elmer Solvent Optimization System (PESOS). These products enabled automated or semiautomated selection of the best solvent to separate a particular sample. The concept of this approach is shown in Figure 2. The methanol–acetonitrile–tetrahydrofuran solvent triangle of Figure 1 is adjusted to an equilateral triangle for convenience. One solvent is chosen to start with and its concentration in water or buffer is adjusted to get retention in the desired range in an effort to achieve a separation, for example, 50:50 methanol–buffer. Next, a second solvent is adjusted to give similar overall retention, for example, a 40:60 acetonitrile–buffer mixture gives similar retention. Then the third solvent is blended with buffer to get similar retention — for example, a 30:70 tetrahydrofuran–buffer mixture in the present situation. By appropriate adjustment of the organic–buffer ratio, the three mobile phases should give very similar retention times when the entire separation is considered — individual retention times are expected to vary between solvent systems. These are experiments 1, 2, and 3 at the corners of the triangle in Figure 2. Next, 1:1 blends are made of the mobile phases from each corner to generate the runs 4, 5, and 6 on the edges of the triangle. Finally a 1:1:1 blend formed the central experiment, 7, to complete the seven-experiment set. Retention data from each of these experiments could be entered into the optimization program and results for conditions between the experimental points could be calculated to find the best possible separation, often a quaternary blend of methanol, acetonitrile, tetrahydrofuran, and buffer.
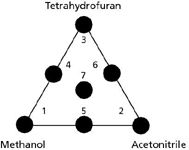
Figure 2: The solvent-selectivity triangle approach to method development.
Alternatively, the seven chromatograms could be printed and set out in the same triangle pattern with visual interpolation to identify the best separation conditions. An example of this approach is shown with the simulated chromatograms of Figure 3. The methanol–buffer run (run 1) separates only four peaks of the five-component mixture — the last two peaks are merged. The acetonitrile–buffer run (run 2) nearly separates the last two peaks, but the second two have merged and reversed. The tetrahydrofuran–buffer run (run 3) has its problems, too — the last two peaks are nicely separated, but the second two are still a problem. It is reasonable to assume that intermediate mobile phase conditions will give intermediate separations when a mobile phase condition is varied. So, if we were making these runs one at a time and examining the results before proceeding, we might skip run 5, because it is obvious from runs 1 and 2 that an intermediate separation of the last two peaks will be no better than for the binary mobile phases. The same prediction could be made for the second two peaks in run 6 based on runs 2 and 3. In run 4, a 50:50 blend of the mobile phases used in runs 1 and 3 is able to separate all five peaks to baseline by giving up part of the excess separation of the first three peaks observed in run 1 and part of the excess separation of the last two peaks from run 3. The quaternary blend of 1:1:1 of runs 1, 2, and 3 to form run 7 is unsatisfactory too.

Figure 3: Simulated partial chromatograms for various mixtures of methanol, acetonitrile, and tetrahydrofuran with buffer. Chromatograms positioned to correspond to Figure 2.
This solvent triangle approach of method development was quite popular in the 1970s and 1980s. It was fairly intuitive to use and generated useful methods. It was equally good for developing isocratic and gradient methods. However, because the mobile phases often comprised all three solvents plus water, they tended to be complex and the more components contained in a mobile phase, the more things can go wrong. This, coupled with the less reproducible nature of the columns of the day, resulted in methods that often needed constant tweaking to keep running. As we'll see in next month's "LC Troubleshooting," further understanding of reversed-phase retention behavior introduced the solvent-strength optimization technique that largely supplanted the solvent selectivity triangle as the most popular approach to method development. But just because it isn't as popular today doesn't mean that we should discard the solvent triangle approach — it is a powerful tool that can help to solve difficult problems, especially after simpler techniques have failed.
Other Information
We've seen how the solvent selectivity triangle helps us understand why acetonitrile, methanol, and tetrahydrofuran have become complementary and preferred solvents for reversed-phase LC mobile phases and how the triangle can serve as an organizational tool for using solvent type to adjust peak spacing in a chromatogram. There are other uses for the solvent triangle as well.
We can use the solvent triangle to help us make wise choices for the selection of additional solvents to test. For example, if we find that methanol in the mobile phase does not give us the peak spacing we need, we might be tempted to try another alcohol — perhaps ethanol or propanol. But the properties of all of these alcohols cause them to be located in the lower left corner of the triangle. This suggests that it is unlikely that we'll find dramatically different chromatographic properties by changing to a different alcohol. We'd be much better moving to an entirely different part of the triangle if our goal is to use solvent selectivity to move peaks around in the chromatogram. On the other hand, there may be other reasons why we might switch from one alcohol to another. For example, larger alcohols, such as propanol, tend to be less denaturing to biomolecules than methanol — it would be reasonable to expect similar, but not identical, chromatographic properties when making this change. As another example, we might find that the basic properties of tetrahydrofuran are favorable for a particular separation, but its other properties, such as relative instability and high UV absorbance, make it less than ideal for some applications. It is possible to successfully substitute another ether, such as methyl tert-butyl ether (MTBE), for tetrahydrofuran in some cases. (MTBE, however, is not fully miscible with water, so it may require a cosolvent in the mobile phase.)
Still another application of the solvent triangle is to identify alternate mobile phase solvents if the supply of solvent is limited. In the alcohol example above, we saw that the properties of the different alcohols were sufficiently similar that a change in alcohol type is unlikely to be an advantage when trying to change selectivity. Conversely, this suggests that we could change from one alcohol to another if necessary for other reasons. For example, if the availability of high performance liquid chromatography (HPLC)-grade methanol became scarce for some reason, we might reasonably switch to ethanol with the hope of obtaining a similar separation. For various economic, political, and practical reasons (see reference 4) HPLC-grade acetonitrile has become quite expensive in the last few years. It would be nice to find another solvent that had similar properties to use as an alternative. Unfortunately, there aren't any readily available solvents in the dipole corner of the triangle that fulfill this requirement, so we are stuck with acetonitrile. If acetonitrile is necessary to get the desired separation and no alternative solvents are available, we have to approach the problem from a different angle. We could change from using a 150 mm × 4.6 mm column to a 150 mm × 2.1 mm column. The reduction in column diameter will allow us to lower the flow rate by a factor of 5 and get the same retention time — for example, from 1 mL/min to 0.2 mL/min. This would reduce the acetonitrile consumption by fivefold, as well. Or we could switch from a 150 mm × 4.6 mm column packed with 5-µm particles to a 100 mm × 4.6 mm column filled with 3-µm particles. Both of these columns will generate approximately 10,000 plates with real samples, so the 100-mm column should reduce the run time by one third — a 15-min run would drop to 10 min, with a corresponding saving of solvent. Or we could combine the two changes and switch from the 150 mm × 4.6 mm, 5-µm column to a 100 mm × 2.1 mm, 3-µm column and reduce the solvent consumption by 5 × 3 = 15-fold. Of course, if these changes were made, we might have to make some other adjustments, such as reducing the amount of injected sample or adjusting the system plumbing to reduce extracolumn volume.
Conclusions
The solvent selectivity triangle helps to simplify the number of solvent choices that are likely to be beneficial to explore during LC method development. It also gives us an organizational framework when exploring solvent selectivity as a means of resolving difficult-to-separate peaks. Finally, we can get some insight into the pros and cons of alternate solvents if we want to make a change. Next month's "LC Troubleshooting" will consider an alternative to solvent-type selectivity: solvent-strength selectivity.
John W. Dolan
"LC Troubleshooting" Editor John Dolan has been writing "LC Troubleshooting" for LCGC for over 25 years. One of the industry's most respected professionals, John is currently the Vice President of and a principal instructor for LC Resources, Walnut Creek, California. He is also a member of LCGC's editorial advisory board. Direct correspondence about this column to "LC Troubleshooting," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail John.Dolan@LCResources.com
For an ongoing discussion of LC troubleshooting with John Dolan and other chromatographers, visit the Chromatography Forum discussion group at http://www.chromforum.org.
References
(1) L. Rohrschneider, J. Chromatogr. 22, 2 (1966).
(2) L. Snyder, J. Chromatogr. 92, 223–230 (1974).
(3) L. Snyder, J. Chromatogr. B 689, 105, (1997).
(4) R.E. Majors, LCGC North America 27(6), 458–471 (2009).

Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.
Potential Obstacles in Chromatographic Analyses Distinguishing Marijuana from Hemp
April 28th 2025LCGC International's April series for National Cannabis Awareness Month concludes with a discussion with Walter B. Wilson from the National Institute of Standard and Technology’s (NIST’s) Chemical Sciences Division regarding recent research his team conducted investigating chromatographic interferences that can potentially inflate the levels of Δ9-THC in Cannabis sativa plant samples, and possible solutions to avoid this problem.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










