Investigating Solvent Purity Using Comprehensive Gas Chromatography: A Study of Acetones
LCGC North America
Investigating Solvent Purity Using Comprehensive Gas Chromatography: A Study of Acetones
General research or specific experimental protocols involving trace and ultratrace chemical analysis and detection is an extremely challenging and daunting task for scientists. As well as the inherent chemical background that is present when a sample is collected, numerous chemical analyses and methods rely on a pure solvent for experimental procedures. For example, many chemical extraction approaches use a solvent to extract target chemicals of interest from a substrate (for example, soil) or collection sorbent. After the extraction step, an evaporation–condensation–concentrating step is performed to reduce the extraction volume to improve detection of trace components. Unfortunately, this volume reduction step will also concentrate many of the impurities present within a solvent and can further confound research efforts. Many of the solvent artifacts introduced into a sample are from chemical impurities that are not completely removed by standard manufacturing processes or techniques for the solvent, as well as the glassware and storage vials used. Regardless of the source, minimizing and understanding these chemical artifacts is critical for trace-level detection and is crucial for unambiguous analytical results.
Acetone is one of the most widely used solvents in the laboratory environment. A common chemical production method for acetone is through the cumene process (1). In the cumene process benzene is alkylated with propene resulting in cumene (isopropylbenzene), which is subsequently oxidized to give phenol and acetone. A wide variety of impurities may exist in the final crude acetone fraction (for example, aliphatic aldehydes, olefins and carbonyl impurities, and more specifically diacetone alcohol, mesityl oxide, and hydroxyacetone), which is subsequently purified to obtain the quoted purity. After the acetone has been obtained in the laboratory, further use or storage may form additional chemical artifacts. Hunchak and Suffet (2) and Lafleur and Pangaro (3) examined acetone–hexane artifacts produced during a Soxhlet extraction process. The chemical artifacts produced through the various procedures and reactions include diacetone alcohol and mesityl oxide as well as phorone, mesitylene, and isophorone (2,3).
The work presented here examines the purity of various acetone sources using comprehensive gas chromatography and mass spectrometry (GC×GC–MS) for broad spectrum chemical analysis. The purpose of this work is to characterize the impurities present in a solvent as a possible approach to track or match acetone sources for chemical forensic investigations. In particular, in the preparation of the toxin ricin, acetone is a common solvent used for the extraction of the castor oil. Previous work developed a simple solid-phase microextraction (SPME) approach to detect the use of acetone as an extraction solvent in the final crude ricin preparation (4). The motivation for this study is to provide additional sample processing information regarding the type or grade of acetone used in the production of ricin or other extracted materials. As broad spectrum chemical analysis becomes increasingly important GC×GC–MS has emerged as a powerful analytical tool. Comprehensive GC×GC is a multidimensional separation technique that separates a sample sequentially on two different columns, which are chosen to separate mixtures based on distinct — ideally orthogonal — chemical properties. Multidimensional separations have the advantage of greatly improved separations compared to conventional single-dimension approaches (5–9). The work presented here is the first step, examining the impurities present in various acetone sources. However, the overall importance of understanding the possible impurities present in a solvent is vital and can be applied to any trace analytical method.

Table I: Acetone source list.
Experimental Section
Chemicals: Table I lists the 21 acetone sources obtained and examined for this study. The mention of trade names or commercial products in this work does not constitute an endorsement, criticism, or recommendation for their use.

Table II: Experimental conditions for chemical analysis.
Chemical Analysis: Chemical analyses were performed on a Leco Pegasus 4D GC×GC–MS system (LECO, St Joseph, Michigan) equipped with a Gerstel cooled injection system (CIS4) and multipurpose sampler (MPS2) (Gerstel, Baltimore, Maryland). The separation used ultrahigh-purity helium as the carrier gas, set at 1 mL/min, constant flow. Because the type and number of impurities were largely unknown for each acetone source, two different comprehensive GC×GC column sets were used (see Table II, A×C and B×C) for all 21 acetone sources. The first dimensional column was either a polar or nonpolar type and the secondary column was of moderate polarity and remained constant throughout the study. Unless stated otherwise the experimental conditions are listed in Table II. LECO ChromaTOF software (version 3.32) was used for data collection and analysis. Higher level data analysis was performed using an in-house software tool (Data Analysis Tool Extension, DAnTE, http://omics.pnl.gov/software/), which is freely available.
Results and Discussion
In this study 21 different acetone sources (brands) were analyzed to examine the trace impurities inherently present within the neat acetone source. Table I lists the 21 acetone sources, which range from high-purity laboratory grade to hardware-store grade to commercial fingernail-polish remover. Within this acetone sample set the supply containers included dark amber glass, clear glass, plastic, and metal. All acetone sources were quoted above 99% purity. In comparison to the extraction solvent artifacts referenced by Hunchak (2) and Lafleur (3), this study attempts to examine the impurities inherently present within an acetone source. To minimize acetone-related artifacts during the analysis, a cooled injection approach is used where 5 µL of acetone is slowly injected into a cooled injection system (CIS) with the majority of injected acetone vented during this process (see Table II for experimental details). Following the acetone injection process into the CIS, the solvent vent is closed and the CIS is rapidly heated to inject the remaining collected residual impurities.
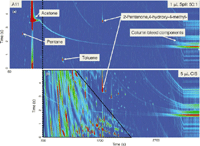
Figure 1: Representative 2D TIC obtained from (a) a 1-µL split injection (split ratio = 50:1) and (b) a 5-µL CIS injection, bottom chromatogram, of acetone A11 (hardware store brand) using the column set of AÃC. Within the chromatograms depicted the blue color represents the baseline signal and the red represents higher signal values. The signal intensity for the two chromatograms is set to 1 à 106 . The black outlined region represents the saturated and unsaturated hydrocarbon compound elution region. The dashed vertical line in the chromatogram (a) denotes the start of data acquisition (that is, 700 s) for the 5-µL CIS injection separation.
To offer a baseline perspective of the purity of a low-grade acetone source, Figure 1 shows a comparison between a 1-µL split injection of moderate split ratio (50:1) and a 5-µL CIS injection of acetone A11 (hardware-store brand) obtained using column set A×C. In these and the following 2D chromatograms, the blue color represents the baseline signal and the red represents higher signal values of compounds. As can be seen in Figure 1, at this 1-µL split injection level (top chromatogram) a number of trace compounds (peaks) are visually apparent, but with acetone the dominant compound in this separation. The major impurity compounds are listed in Figure 1 and include 2-pentanone, 4-hydroxy-4-methyl- (diacetone alcohol), and toluene. Pentane is observed as carryover from the syringe wash solvent used between injections. The horizontal solute bands at the end of the chromatogram are due to column bleed components. In general even this low-grade acetone source appears to be relatively pure at this injection level. However, when a 5-µL CIS injection of the same A11 is examined the number of impurities observed dramatically increases as shown in the lower chromatogram in Figure 1, where the vertical dashed line denotes the start of the data acquisition for the larger injection volume. These impurity components encompass a wide range of polarity, volatility, and analytical stability. Depending upon the source and type of acetone, numerous trace impurities are found in all 21 acetone sources and all the sources can easily be differentiated by visual inspection of the chromatograms.
Figure 2 summarizes the separations obtained for five of the acetone sources (A3, A9, A20, A23, and A27) for both column sets (that is, A×C and B×C) acquired using the 5-µL CIS injection approach. A number of observations are illustrated in Figure 2. In general, among all the acetone sources the number and amount of impurities can be extremely large and variable. The general chemical classes observed include saturated and unsaturated hydrocarbons, organic acids, ketones, and branched aromatic hydrocarbons according to their chromatographic elution order and mass spectral information. Interestingly, regardless of the column set used (that is, A×C or B×C) various solute zones appear as large streaks typically early in the 2D chromatograms, which was also observed in Figure 1. This solute zone streaking is probably due to the chemical mismatch between the solute and stationary phase (for example, nonpolar solute and a polar stationary phase and vice versa) of the first dimensional column so that there is a lack of adequate solute focusing during the injection process. For example, in acetones A3 and A9, both acetic acid and propanoic acid are found by using column set A×C with good solute zone shape; however, acetic acid is absent within the data collection window used and propanoic acid appears as a large streak when using column set B×C (Figure 2). Obviously the presence of these trace acids in the solvent could have deleterious effects for some solutes (for example, reactions producing artifactual solutes) and could confound trace-level applications. Importantly, these organic acids could easily go undetected if only a nonpolar-type column were used. Conversely, many of the other acetone sources have a large number of what appear to be hydrocarbon-type impurities according to the mass spectral information. These hydrocarbon-type compounds appear as streaks early in the 2D chromatograms when using column set A×C (for example, acetone sources A9 and A27 in Figure 2, as well as A11 in Figure 1 as highlighted by black outlined regions), but they appear as narrow solute zones with column set B×C. This hydrocarbon source region is outlined for acetone source A9 in Figure 2 and contains numerous straight chain, branched, and cyclo hydrocarbon compounds as indicated by their electron impact mass spectra. Varying amounts of these hydrocarbons can be found in all the acetone sources examined and the solute zone shape is more clearly defined when using column set B×C. Again, these solutes may go undetected if using column set A×C due to the lack of solute focusing or streaking. This result highlights the importance of using a number of different primary columns of different chemical nature when examining for trace-level components in a broad spectrum chemical analysis. (Note: As an aside, when using a common second dimensional column and changing only the first-dimension column the down time of the GC×GC–MS instrument is minimal, as the mass spectrometer may not need to be vented during this column switch.)
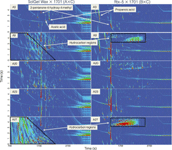
Figure 2: Representative 2D TIC comparing the impurities observed for five of the acetone sources (A3, A9, A20, A23, and A27) for both column sets (AµC and BµC) and a 5-µL CIS injection. The black outlined areas depict the elution region of the saturated and unsaturated hydrocarbon compounds. The difference between the elution regions and the solute zone shape for the two different column sets AÃC and BÃC is illustrated. The signal intensity for the chromatograms is set to 1 à 106
The hydrocarbon region outlined in A9 in Figure 2 is examined more closely in Figure 3 with a comparison between acetone sources A9 and A27. In comprehensive 2D separations compared to conventional 1D GC separations, chemically similar compounds will create patterns or groupings of solute zones (for example, a homologous series of saturated hydrocarbons) within the 2D separation plane. These chemical groupings or families in a 2D chromatogram can be of help in identifying unknowns, when no standard components are available, in the absence of commercial MS library spectra or when the resulting experimental MS spectra are very similar to others. In Figure 3, three m/z values are selected to highlight groupings of saturated hydrocarbons (m/z = 57), olefins (m/z = 69), and alkyl substituted benzene rings (m/z = 91), which also captures possible mesitylene, cumene, and their isomeric impurities. In general, as expected, the A9 acetone source appears to be of higher chemical purity than A27 as depicted by the smaller number of solute zones. Interestingly, acetone source A9 also appears to have a greater fraction of saturated and unsaturated hydrocarbon compounds that are eluted later in time. This implies that A9 has a larger fraction of higher boiling point hydrocarbons than does A27, which may indicate a certain type or degree of purification process. Finally, a series–family of alkyl substituted benzyl compounds (m/z = 91) is observed in both acetone sources, but the amounts present in acetone A27 are much larger. Again the presence of these benzyl compounds may indicate the type or extent of the purification process used.
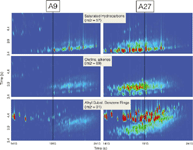
Figure 3: Magnified chromatographic region outlined in Figure 2 for acetone sources A9 and A27, column set BÃC. For clarity reconstructed SICs are selected to highlight groupings of saturated hydrocarbons (m/z = 57, signal intensity = 5 Ã 105 ), olefins (m/z = 69, signal intensity = 5 Ã 105 ) and alkyl-substituted benzene rings (m/z = 91, which captures possible meitylene, cumene and their isomeric impurities, signal intensity = 5 Ã 104 ).
All 21 acetone sources were found to contain four of the common acetone impurities expected; diacetone alcohol (2-pentanone,4-hydroxy-4-methyl-), mesityl oxide (3-penten-2-one,4-methyl-), phorone (2,5-heptadien-4-one,2,6-dimethyl-), and phenol, but at varying amounts. Figure 4 illustrates a heatmap plot summarizing the results obtained from these four compounds for the 21 acetone sources using the A×C column set. In Figure 4, the solute zone volumes (absolute detector counts) are based upon the average of three replicate injections of the acetone source, which were analyzed in a random order. The average solute volumes illustrated in Figure 4 are calculated based on a log10 scale, where the blue color represents the lowest signal intensity and the red color represents the largest signal intensity of the compound. As illustrated in Figure 4 there appears to be a large variation in the amount of these four compounds for each acetone source. This indicates that acetone CIS injection process is not contributing appreciably to the presence of these compounds and that the components are likely residual from the manufacturing or distribution process. From Table I, acetone sources A23, A24, and A25 are from the same distributor and the same lot (that is, different bottles); acetone sources A9 and A10 are from the same distributor, but different lots (this is also true for acetone sources A5 and A6; however, A5 displayed a large isopropyl alcohol impurity of approximately 1:1 relative to acetone, chromatogram not shown); acetone sources A11, A27, and A28 are commonly available hardware store brands. These "source" trends are also observed in Figure 4, where these acetone sources tend to cluster closer to each other. The exception maybe the closer clustering of A9 and A20 to each other compared to the A9 and A10. Again this clustering is only using these four expected impurities, where the actual number of compound impurities is extremely large. As shown in Figure 2 the number, type, and amount of impurities present between these two sources (A9 and A20) is very different.
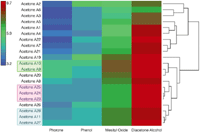
Figure 4: A heatmap plot summarizing the results obtained using the signals from diacetone alcohol (2-pentanone,4-hydroxy-4-methyl-), mesityl oxide (3-penten-2-one,4-methyl-), phorone (2,5-heptadien-4-one,2,6-dimethyl-) for the 21 acetone sources using the AÃC column. The default settings for heatmap construction were used. This allows hierarchical clustering through a complete linkage of the Euclidean distance.
Conclusion
For broad spectrum screening or trace component detection against complex background mixtures, impurities present within a solvent can significantly confound research efforts. In this work comprehensive GC–MS is used to assess the impurities present in various commercial acetone brands. A large number and broad range of different chemical impurities were found, dependent upon the acetone source. These chemical impurities ranged in complexity from simple organic acids, alkanes, and olefins, to a variety of substituted benzyl compounds (to highlight just a few). In general, dependent upon the analytical questions trying to be answered, a chemist should not become complacent and should be cautious of the impurities that may be present (and how they may change with different brands) and interfere with a trace-component analysis. This is highlighted by the presence of the organic acids present in various acetone sources and the subsequent need for two different column sets to better capture and understand the range of impurities present. Moreover this work addresses only those compounds that are stable and volatile for the GC system and does not address those components more labile or nonvolatile that may also be present in a solvent to alter or affect results.
Acknowledgments
Funding for this work was provided through contract AGRHSHQDC07X00451 to Pacific Northwest National Laboratory by the United States Department of Homeland Security, Science and Technology Directorate.
Jon Wahl is a senior research scientist in the Chemical and Biological Signature Sciences Group at Pacific Northwest National Laboratory. He has a PhD in Analytical Chemistry from Michigan State University and a BS in Chemistry from Michigan Technological University. Dr Wahl's principal research interests focus on interdisciplinary chemical and bioanalytical problems that require advanced high-resolution separation techniques. Multidimensional separations for ultratrace analysis methods for chemical warfare agents and related compounds, biological, explosives and other signature compounds of interest are a main focus.
Cinnamon Bolz received a BS in Biochemistry from Portland State University and a MS in Forensic Science from National University. She has experience in a variety of analytical techniques including separations, mass spectrometry, and spectrophotometry in addition to writing standard operating procedures and quality control manuals, and applying statistical data analysis.
Karen Wahl is a staff scientist within the Chemical and Biological Signature Sciences Group at Pacific Northwest National Laboratory in Richland, Washington. Dr Wahl has a PhD in Analytical Chemistry from Michigan State University and a BA in Chemistry from The College of Wooster. Dr Wahl's research interests include the development of MALDI mass spectrometry for microorganism analysis and the development of analytical methods for chemical and biological forensics.
References
(1) V.J. Aristovich et al., Method for Purifying Acetone, US Patent 6340777.
(2) K. Hunchak and I.H. (Mel) Suffet, J. Chromatogr. 392, 185–198 (1987).
(3) A.L. Lafleur and N. Pangaro, Analytical Letters 14(A19), 1613–1624 (1981).
(4) H.W. Kreuzer et al., J. Forensic Sci., in press.
(5) P.J. Marriott and R.A Shellie, Trends Anal. Chem. 21, 573–583 (2002).
(6) J. Beens and U.A.Th. Brinkman, Analyst 130, 123–127 (2005).
(7) M. Adahchour et al., Trends Anal. Chem. 25, 540–553 (2006).
(8) M. Adahchour et al., Trends Anal. Chem. 25, 726–741 (2006).
(9) M. Adahchour et al., Trends Anal. Chem. 25, 821–840 (2006).

Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).
Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








