A Review of Column Developments for Supercritical Fluid Chromatography
LCGC North America
The authors review the requirements for columns specifically designed and manufactured for SFC.
Supercritical fluid chromatography (SFC) has a convoluted past. On the instrumentation side, academic laboratories have found it difficult to make or buy SFC equipment, resulting in most development being made by end users or manufacturers. The history of column development closely follows the history of SFC instrumentation. Column manufacturers have been slow to embrace SFC. In fact, up until about 2000, there were few SFC-specific columns. So perhaps the most important aspect of this article is the fact that there is actually something to review. Because this is a maiden attempt to review the role of columns for SFC, we first present a very brief history of commercial SFC instrumentation, followed by the closely related evolution of thought on how to make SFC columns, and concluding with what's available today (and why). There is no attempt to be comprehensive.

Terry Berger
The Evolution of Instrumentation for SFC
Klesper first demonstrated SFC in 1962 (1), collecting fractions and analyzing them off-line. Widespread awareness of SFC only occurred after Hewlett Packard (HP, now Agilent Technologies, Santa Clara, California) presented a series of papers at the 1979 Pittsburgh Conference and introduced an SFC modification kit for the model 1084 high performance liquid chromatography (HPLC) system, in 1981. This was a packed-column instrument with independent flow, composition, pressure, and temperature control. Detection was by UV, but other detection methods, such as flame ionization detection (FID) and mass spectrometry (MS) were sometimes used. Columns of the day were standard normal-phase columns borrowed from HPLC. The common packings were silica, phenyl, cyano, amino, and diol. Few were endcapped. Most were 250 mm × 4.6 mm with 5-μm totally porous silica packings, but the range of lengths, internal diameters, and particle sizes available were similar to today, including a few experimental sub-2-μm pellicular–nonporous packings. Elution was isocratic or with composition gradients. The fluid of choice was carbon dioxide, modified with an organic solvent. The back-pressure regulator was a slightly modified mechanical type, but a few were further modified with a chain drive and a stepper motor for pressure programming. When the SFC-incompatible HP model 1090 HPLC system was introduced, the HP model 1084 was withdrawn from the market, ending production of the first commercial SFC system.

Blair Berger
Preparative SFC had a beginning in the early 1980s with Perrut patenting recycle preparative SFC with cyclone separators, for petroleum applications, using pure carbon dioxide as the mobile phase, in 1982 (2). Manufacturers Prochrom [now part of Novasep (Pompey, France)] and Novasep have had a continuous presence in larger scale SFC since that time. Jasco (Easton, Maryland) introduced their combined supercritical fluid extraction (SFE)–SFC system in 1985, which was similar to the modified HP1084 system. It featured the first electronic back pressure regulator specifically designed for SFC (and SFE).
Capillary or open-tubular SFC, originally reported by Milton Lee and others in 1984 (3), was commercialized by 1986, through Lee Scientific (acquired by Dionex Corporation, Sunnyvale, California) but spun off. Core individuals are now part of Selerity (Salt Lake City, Utah). Capillary columns dominated the development of both theory and instrumentation in SFC for the next 5–7 years. Capillary instruments closely resembled a GC, but used a syringe pump as a pressure source, and a fixed restrictor to limit flow through small-internal-diameter open-tubular columns. The vast majority of applications used pressure programming with pure carbon dioxide as the mobile phase. Detection was FID but many other detectors were used, including MS. Columns were mostly 50-μm i.d. fused silica.

Ronald E. Majors
Through the late 1980s, numerous other companies (including Isco [Lincoln, Nebraska], Carlo Erba [Milan, Italy], and CDS [Avondale, Pennsylvania]) introduced similar equipment (syringe pumps, pressure programming, fixed restrictors, and FID systems), but they were limited by a U.S. patent to only using micropacked columns. Most have exited the business and some are no longer in business or have been absorbed by others.
In 1992, Gilson and HP both introduced commercial analytical-scale SFC systems. For HP, this was their second product entry. Their hardware used binary, reciprocating pumps, electronic back pressure regulators, UV detectors, and computer control of all variables. The HP system was capable of both packed and capillary column operation. The Gilson pumps delivered a higher flow rate, prompting several largely unsuccessful attempts to create a small scale semipreparative instrument, using 1-cm columns. Gilson exited the SFC business around 2002. The HP product line was sold to Berger Instruments (BI) in 1995. BI immediately dropped capillary operation.
Semipreparative SFC took off after BI introduced the semipreparative AutoPrep system for achiral library purification and the MultiGram II system for chiral-like stacked injections. These were basically scaled up versions of the analytical hardware, with a new kind of phase separator. Columns were 2–3 cm i.d., with packings identical to analytical columns. BI was acquired by Mettler Toledo in 2000, who sold the business to Thar (2007), who were acquired by Waters (Milford, Massachusetts) (2009). Agilent Technologies is reentering the business (2010), working with Aurora SFC Systems (Sunnyvale, California), to convert certain HPLC systems into SFC systems with minimal modification. Thus, for the first time, the two main suppliers of HPLC equipment (Waters and Agilent) are both involved in SFC.
Column Development
The turbulence in SFC hardware development during the 1980s through the 1990s can be directly attributed to an unfortunate elutropic series published by Calvin Giddings (4 ). Giddings estimated that dense carbon dioxide was similar to isopropanol in solvent strength. This implied that density programming of pure carbon dioxide would have the same effect as a composition program from pure hexane to pure isopropanol. This would have been hugely important, as controlling a physical parameter, such as pressure, is far easier and less expensive than controlling flow and composition. It also would allow the use of FID. With the benefit of hindsight, we must say this eluotropic series is completely wrong. The Giddings series was never challenged publicly and was never corrected. No one would suggest using pure hexane to separate polar drugs, yet (we now know that) pure carbon dioxide, a very nonpolar substance, similar to a hydrocarbon, was presumed to elute such compounds.
Low-to-moderately polar compounds such as benzoic acid and aniline could be eluted from capillary columns with pure carbon dioxide, whereas they exhibit much more retention, tailed severely, or could not be eluted from packed columns under the same conditions. The elution from capillaries was thought to confirm a high solvent strength for the pure carbon dioxide. The poor performance by the packed columns was widely attributed to "active sites" on the columns. Capillary columns have a much lower phase ratio than packed columns and typically are coated with less polar phases. Each characteristic makes capillaries much less retentive than packed columns, but these facts were largely ignored.
Several reviews (5,6) of packed columns for SFC, written in 1990, indicate a near obsession with the use of pure fluids, pressure programming, and FID. It was still widely thought that modifiers only covered active sites on the support, and did not increase solvent strength dramatically, or improve the solubility of polar solutes. These can be summarized by the following statement: "Therefore, stationary phases for SFC are desirable which allow the elution of the largest possible variety of solutes as sharp, symmetrical peaks, using pure carbon dioxide . . ." (6). Thus, creating a more inert support was thought to be the way forward.
Polymer-coated particles such as Deltabond (Keystone Scientific, now part of Thermo Fisher Scientific, Madison, Wisconsin) allowed the elution of somewhat more polar molecules and somewhat improved peak shapes, but the degree of improvement was still limited, often producing tailing peaks. Several polymer-based particles were also developed, without silica. These tended to allow the elution of slightly polar compounds (aniline, benzoic acid), without additives, but efficiency was very poor. Unfortunately, these modest improvements further encouraged the use of pure carbon dioxide, with more polar solutes, while increasing the resistance to the more complex and expensive use of binary pumping systems, and UV detectors with packed columns.
Throughout the 1980s and into the 1990s, a relatively small number of laboratories continued to use modified HPLC systems with binary pumping systems, composition programming, and UV detection. The use of additives was pioneered in the very late 1980s (7–11), which dramatically increased the polarity of compounds that could be separated by SFC (12–16). Many classes of polar solutes including phenols, polyhydroxy, hydroxyacids, polyacids, aliphatic amines, and many drug families were only separated using additives like citric acid, trifluoroacetic acid, isopropylamine, triethylamine, ammonium acetate, and many others.
By 2000, it was acknowledged that capillary SFC had been oversold (17) especially for the elution of polar solutes. Nevertheless it was still stated that: "Although the future will undoubtedly see continued greater use of packed column than open-tubular column SFC, the two techniques should be seen as complementary rather than competitive methods."
It also was stated that packed column SFC was a "suitable replacement for normal-phase liquid chromatography." This simple statement fails to convey the tremendous advantages SFC has versus normal-phase HPLC. Normal-phase LC mobile phases are largely limited to nonpolar (such as hexane and isooctane) and highly flammable organic mixtures. Traces of water in the mobile phase caused significant shifts in retention times. Reequilibration times are very long. Solute binary diffusion coefficients are significantly lower than in modified carbon dioxide, making the chromatography much slower. Viscosity of the fluids is higher, leading to larger pressure drops. SFC is much faster, reequilibrates extremely fast, allows steep gradients, is tolerant of significant amounts of water, allows very long columns, or very small particles with modest pressure drops, is not flammable, and is "green." In our opinion, SFC outperforms normal-phase HPLC and even reversed-phase HPLC.
Before 2000, there had been few columns sold only as SFC columns. An example is the hydrocarbon group separation columns used to measure aromatics in diesel and olefins in gasoline. These were nothing more than conventional bare silica columns with low metals content (Type B silica), sometimes used with a silver-loaded stationary phase or an amino phase to further separate the olefins from the saturates.
Commercial column manufacturers tended to ignore SFC until the market grew large enough to justify an investment. Consequently, by 2000 most of the available polar stationary phases were the same as those used more than 20 years earlier in normal-phase HPLC, with little improvement. Also, they did not provide a large variation in selectivity.
By the end of 2000, there was a general perception that SFC could separate a large percentage of small drug-like molecules, but that additives were generally necessary. In fact, additives were used so ubiquitously that often they were included in the mobile phase (8,18), even when they weren't necessary (19)! Additives continue to be viewed as undesirable, particularly when a mass spectrometer is used as the detector. In one instance, an isopropylamine additive reacted with a ketone solute. On the other hand, another ketone solute appeared to react with an amino stationary phase in the absence of an additive. In spite of this, SFC has been moving vigorously into routine achiral analysis (20–23).
Particle Size and Speed in SFC
In the early 1980s, much of the work published by Dennis Gere (24) used 3-μm particles. Amazingly, there has been a subsequent, surprising dearth of publications using smaller particles in SFC. This absence is all the more surprising when considering the recent major emphasis in HPLC for particles smaller than 2 μm. The diffusion coefficients of solutes in carbon dioxide are 3–5 times higher than in HPLC, so SFC should be 3–5 times faster on the same-sized particles. Perhaps, more importantly, the viscosity of the fluids is much lower than aqueous based mobile phases. Consequently, pressure drops are much lower, even with the higher linear velocities. Thus, SFC on 3 μm particles should be as fast or faster than HPLC on sub-2-μm particles.
To illustrate the speed potential for SFC by variation of particle size and column length, several chromatograms were prepared, as there were few similar examples available in the literature. It often is desirable to transfer a method from a long column packed with larger particles to a shorter column with smaller particles, in order to save analysis time. For method transfer to be feasible, the stationary phase must remain consistent independent of particle size or pressure drops. Three different particle sizes of the same base silica and three different column lengths were compared at the same column internal diameter and isocratic conditions at 50 °C (see Figure 1). The outlet pressure was fixed at 150 bar, and the flow rate was varied between 2 and 5 mL/min, depending on particle diameter. Figure 1a shows the separation of xanthenes and profens using a 250 mm × 4.6 mm column packed with 5.0-μm silica. The last peak exhibited over 25,630 isocratic plates in about 4 min. Decreasing particle size and column length (Figure 1b) keeps the resolution about the same but cuts the analysis time to 1.5 min, just as in HPLC. A further reduction in column length while keeping the particle size the same (Figure 1c), results in a separation of about one third of the time but with a slight loss in resolution and efficiency. Finally, use of a sub-2-μm particle in an equivalent length column (Figure 1d) results in a further reduction in analysis time (0.3-min) with some further band broadening due to two causes. First, the 5-mL/min flow rate on the latter chromatogram (Figure 1d) was significantly suboptimum (below the van Deemter minimum) for a 4.6-mm i.d. column and was limited by the 5-mL/min capability of the pump. Second, the model 1100B diode-array detector had a maximum data rate of 20 Hz and therefore the chromatographic efficiency of the faster columns was compromised by its slower than required response. The chromatograms throughout show very consistent selectivity, even though the particle diameter was decreased from 5 μm to 3.5 μm, and then to 1.8 μm.
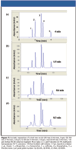
Figure 1
SFC can accomplish the same or greater speed compared to UHPLC, using conventional (400 bar) HPLC hardware and columns, because the viscosity of the fluids is dramatically lower, resulting in lower pressure drops.
HILIC SFC Columns
Hydrophilic interaction liquid chromatography (HILIC) is an extension of normal-phase HPLC to more polar solutes. It has been around since the 1980s, and is characterized by a hydrophobic, mostly organic (low water content) mobile phase used with a hydrophilic stationary phase in which ionic additives, like ammonium acetate, are often used. It is thought that a thin film of adsorbed water acts as part of the stationary phase. This is similar to SFC, in which it has been shown that polar modifiers and additives preferentially adsorb (25,26) onto polar stationary phases to form multiple monolayers. Surprisingly, the literature contains very few references (27) to the use of HILIC columns in SFC.
Superficially Porous Particles for SFC
Another recent trend in HPLC is the use of small, solid-core (superficially porous) particles coated with a thick porous layer of silica (28–32). These particles generate higher efficiencies compared to totally porous silica of the same particle diameter. Guiochon (33) recently proposed a theory covering efficiency in reversed-phase HPLC for such particles. There appears to be no reference in the literature available to the author, in which such columns have been used in SFC. However, HILIC versions are available commercially. The chromatogram in Figure 2 was generated using a Phenomenex Kinetex HILIC column packed with a 2.6-μm superficially porous particle and dimensions of 150 mm × 4.6 mm. In this chromatogram, up to 26,000 plates and nearly 1100 plates/s were generated. In a somewhat slower chromatogram, up to 50,000 apparent plates were generated in isocratic runs. The reduced plate height was as low as 1.3. These somewhat anecdotal results suggest there is a very exciting path forward in SFC, and the author hopes others will publish more using such columns.
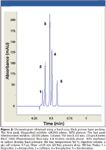
Figure 2
Long Columns for High Resolution SFC
Another subtle trend in SFC is in the use of longer columns. In the late 1980s there were competing theories (34–38) as to why pressure drops along the axis of packed columns caused excessive loss of efficiency. In fact, it was stated (36) that packed columns could not generate more than approximately 20,000 plates. This perception was overturned when Berger (39) generated 220,000 plates on a 2.2-m-long column packed with 5-μm particles, with a column hold up time (void time) of 12 min. In some circumstances, the only effective means of resolving complex mixtures or difficult to separate pairs is simply the brute force approach of increasing the number of plates by increasing column length. The low viscosity and high diffusivity allows the use of longer columns with modest pressure drops.
Kot (40), and Phinney (41) extended the concept by connecting a series of as many as five different chiral phases in series, to create a pseudo-universal chiral column, or combine achiral and chiral columns to adjust selectivity in mixtures.
Recently, the concept was revived when five 25-cm-long cyano columns were connected in series to provide a high-resolution separation in complex pharmaceutical analysis (42). The column stack produced approximately 100,000 plates and was used with composition gradients.
Chemometrics and Phase Selectivity
With the recent rapid growth of SFC, there has been an explosion of interest in various chemometric approaches to predict retention, although the first attempts date back to the early 1990s (43). Lessellier and West have been particularly active (44–48). In recent work (49), they presented a graphical comparison of the selectivity of a large number of different stationary phases along five axes, each representing a different solvation parameter, as shown in Figure 3. Not too surprisingly, almost all the traditional phases (amino, cyano, diol, and silica) are bunched together, showing mostly strong hydrogen bond acidity interactions. Perhaps slightly surprisingly, ethyl pyridine is very similar, located between cyano and amino. Diol is shown to be slightly more affected by hydrogen bond donor basicity than the others.

Figure 3
Another group (50) used linear solvation energy relationships (LSERs) with 200 test solutes and came to a slightly different conclusion stating that hydrogen bond donor acidity of the solutes is particularly important for pyridine and amino columns. Hydrogen bond donating ability is small for cyano and pyridine stationary phases (as one might expect). Hydrogen bond acceptor basicity of the solutes is particularly important for diol and amino columns.
Yet another approach (51) correlated the retention of some sulfonamides with total dipole moment, molecular surface area, and the "electronic charge on the most negatively charged atom." Above 10%, log k was shown to vary linearly with % modifier.
Commercial Achiral Analytical- to Semipreparative-Scale Columns
Today, the "holy grail" of SFC column research remains the elution of polar solutes without the use of very polar additives, with at least three companies creating new phases for SFC. In 2001, Princeton Chromatography (Cranbury, New Jersey) introduced the 2-ethylpyridine stationary phase bonded on totally porous silica. This was possibly the first stationary phase specifically designed for packed column SFC. Many compounds, particularly amines, can be eluted with no additive, although one is still sometimes required.
The introduction of this phase initiated a fairly consistent, progressive development of a number of phases both by Princeton and others with the intent to decrease tailing and providing alternative selectivities. This new interest in SFC column development has involved primarily smaller companies like Princeton and ES Industries (Berlin, New Jersey), but Phenomenex (Torrance, California), one of the larger column manufacturers, has also created an SFC product line. A few others make a modest number of older phases.
Princeton Chromatography also makes the more traditional amino, cyano, diol, and silica columns along with a number of newer phases including a high-load diol (Diol HL), 2- and 4-ethylpyridine, pyridine amide, diethylamino (DEAP), propyl acetamide (PA), and others. Most columns are packed with 3- or 5-μm particles. Princeton appears to be unique in using an SFC instrument to measure the performance of every column shipped. They also provide matched sets of analytical and semipreparative columns using the same batch of packing material.
ES Industries also sells a complete line of traditional amino, diol, cyano, and silica SFC phases, including NO2, DEAP, and PFP (pentafluorophenyl). Their newer phases include amino phenyl, ethyl pyridine, pyridyl amide, "Epic" Nitro, and FluoroSep Phenyl as SFC columns. On their website, they talk about separating amines, alcohols, and acids without the use of additives on the amino phenyl phase and amines without amino additives on ethyl pyridine. They also claim simplified fraction collection without amine or trifluoroacetic acid additives using the pyridyl amide phase. ES Industries also makes 1.8-μm particles coated with amino, phenyl, ethyl pyridine, and FluoroSep phenyl.
Phenomenex sells silica, cyano, and amino traditional phases specifically designed for SFC, along with several newer SFC phases including PFP(2) (pentafluorophenyl) and a HILIC column all under their Luna brand. They also sell a Synergi Polar-RP phase, which is an ether-linked phenyl with additional hydrophylic endcapping. The company claims the PFP phase employs hydrogen bonding, dipole, and aromatic interactions in addition to hydrophobic interactions to dramatically change selectivity compared to alkyl phases such as C18.
Their hard-core thick porous-layer particles under the brand Kinetex can generate efficiencies in very short times as shown in Figure 2. They also make sub-2-μm particles for SFC.
Akzo Nobel (Bohus, Sweden) supplies Kromasil with all the achiral traditional phases (amino, cyano, diol, phenyl, and silica) and mentions their use in SFC. Kromasil has been used extensively in SFC but mostly as a base silica material with chemistries applied by others.
Restek (Bellefonte, Pennsylvania) makes a wide range of appropriate phases such as amino, cyano, PFP, phenyl, and silica, but it does not promote them for use in SFC. Supelco offers a number of phases through Sigma-Aldrich (St. Louis, Missouri) including amino, cyano, a polar embedded amide, PFP, phenyl, silica, and HILIC, but it does not mention their use in SFC. Thermo Fisher Scientific sells Hypersil cyano, PFP, phenyl, and a polar end-capped C18 (aQ), but it does not mention or promote SFC. Grace Davison (Baltimore, Maryland) does not mention SFC.
At Pittcon 2010, Waters announced a line of Viridis SFC columns from analytical to semipreparative, concentrating on semipreparative. Initially, a 2-ethylpyridine phase and silica were introduced in dimensions of 4.6–30 mm i.d. and 50–250 mm lengths. Agilent's offerings are limited to silica, cyano, HILIC, and Poroshell 120/300 superficially porous shell columns. With their entry into the SFC hardware manufacturing market, both Waters and Agilent are likely to extend the number of phases offered in the future.
Commercial Chiral Analytical- and Semipreparative-Scale Columns
Chiral method development, enantiomeric excess determinations, and chiral semipreparative separations have been the strongest applications of SFC, having significantly penetrated every major pharmaceutical company (52–56). A review of applications through 2008 is available (57).
The discussion of chiral stationary phases (CSPs) will be brief, as most of what is new is also discussed in HPLC. The phases based upon macrocrystalline cellulose and amylose continue to be the most popular. Chiral Technologies (West Chester, Pennsylvania) reports that over 85% of the enantiomeric separations in the literature are achieved on one of six such CSPs developed by them for both HPLC and SFC. In the standard 250 mm × 4.6 mm format, they offer the six most popular CSPs on 5-μm particles, plus a 2.1-mm internal diameter and a 150-mm length. Chiral Technologies developed 100-mm-long columns, with 5-μm packings, specifically for rapid screening by SFC. They have also introduced two Lindner phases on 5-μm silica. Finally, they have also introduced three bonded (immobilized) phases ChiralPak IA, IB, and IC, with the same selectors as AD, OD, and a unique phase. They are now selling prepacked columns with internal diameters as large as 5 cm.
With the expiration of several key patents, several companies have introduced the equivalent of OD and AD, in a few new formats. Phenomenex sells just those two chiral phases, in 3, 5, and 10 μm, and specifically recommends them for SFC. Regis Technologies (Morton Grove, Illinois) offers similar columns, and supports SFC. Of course Regis also continues to sell many other stationary phases, including both enantiomers of Pirkle 1-J, Whelk-O1, Whelk-O2, Burke2, beta-GEM1, and others. Akzo-Nobel offers their same DBM and TBB phases plus an OD and AD equivalent in 3–25 μm particles.
Astec (now part of Supelco/Sigma Aldrich) still offers phases based upon macrocyclic glycoproteins, beta and gamma bonded cyclodextrins, and a polymerized cyclic diamine, which have all been used in the past for SFC. Astec only mentions "normal phase."
Conclusions
The turmoil of the past appears to be over. The path forward seems clear. SFC is finally stepping out of the shadow of HPLC and seems destined to experience robust growth in the near future. Chiral separations still dominate, but SFC is not just for chiral anymore. The inherent speed and resolution of SFC is being recognized by a much wider base of chromatographers. There is finally an active effort on the part of several column companies to create better columns with enhanced selectivities, specifically designed for SFC. The strong trend in achiral column development is fueled by an increasing awareness that the high speed and efficiency, the orthogonal selectivity, the low-pressure drops, low operating cost, and the low environmental impact, are general phenomena, inherent to SFC. SFC appears poised to enter the area of validated methods (QA, QC, PV, other trace analysis) in a big way. The involvement of the two largest HPLC hardware manufacturers can only help increase the awareness and acceptance of the technique.
Terry Berger and Blair Berger are with Aurora SFC Systems, Inc., Sunnyvale California. Terry Berger has been active in SFC since the early 1980s, first at Hewlett Packard, then Berger Instruments, and is now active in Aurora SFC Systems, Inc. Terry was awarded the Martin Gold Metal in 2004, by the Chromatographic Society for his contributions to chromatography. Blair Berger graduated from University of Tampa in 2007 with a B.S. in Marine Science, a BS in Biology and a minor in chemistry. Blair has intermittently run SFC instrumentation since 2001, and has been involved with Aurora SFC Systems since its beginnings and has been working as a research assistant for Aurora SFC for six months. Direct correspondence to: tberger@aurorasfc.com.
Ronald E. Majors "Column Watch" Editor Ronald E. Majors is Senior Scientist, Columns and Supplies Division, Agilent Technologies, and is a member of LCGC's editorial advisory board.
References
(1) E. Klesper, A.H. Corwin, and D.A. Turner, J. Org. Chem. 27, 700–701, (1962).
(2) M. Perrut, U.S. Patent No. 4,478,720, Oct. 23, 1984.
(3) M. Novotny, S.R. Springston, P.A. Peaden,, J.C. Fjeldsted, and M.L. Lee, Anal. Chem., 53, 407A (1981).
(4) J.C. Giddings, M.N. Meyers, L.M. McLaren, R.A. Keller, Science 162, 67 (1968).
(5) M. Petersen, J. Chromatogr. 505, 3 (1990).
(6) P.J. Schoenmakers, L.G.M. Uunk, and H-G Janssen, J. Chromatogr. 506, 563 (1990).
(7) M. Ashraf Korassani, M.G. Fessahaie, L.T. Taylor, T.A. Berger, and J.F. Deye, J. High Resolut. Chromatogr. 11, 352 (1988).
(8) W. Steuer, M. Schindler, G. Schill, and F. Erni, J. Chromatogr. 441, 287 (1988).
(9) T.A. Berger, J.F. Deye, M. Ashraf-Korassani and L.T. Taylor, J. Chromatogr. Sci. 27, 105 (1989).
(10) T.A. Berger and J.F. Deye, J. Chromatogr. Sci. 29, 310 (1991).
(11) T.A. Berger and J.F. Deye, J. Chromatogr. 547, 377 (1991).
(12) T.A. Berger and J.F. Deye, J. Chromatogr. Sci. 29, 54 (1991).
(13) T.A. Berger and J.F. Deye, J. Chromatogr. Sci. 29, 26(1991).
(14) T.A. Berger and J.F. Deye, J. Chromatogr. Sci. 29, 141 (1991).
(15) T.A. Berger and J.F. Deye, J. Chromatogr. Sci. 29, 310 (1991).
(16) T.A. Berger and J.F. Deye, J. Chromatogr. Sci. 29 127 (1993).
(17) C.F. Poole, J.Biochem. Biophys. Methods 43, 3 (2003).
(18) O. Gyllenhaal and J. Vessman, J. Chromatogr., A 839, 141 (1999).
(19) J. Lundgren, J. Salomonsson, O. Gyllenhaal, and E. Johansson, J. Chromatogr., A 1154 360, (2007).
(20) C. White and J. Burnett, J. Chromatogr., A 1074, 175 (2005).
(21) B. Bola, M. Greig, M. Ventura, W. Farrell, C.M. Aurigemma, H. Li, T.L. Quenzer, K. Tivel, J.M.R. Bylund, P. Tran, C. Pham, and D. Phillipson, Int. J. Mass Spectrom. 238, 85 (2004).
(22) R.A. Coe, J.O. Rathe, and J.W. Lee, J. Pharma. Biomed. Anal. 42, 573 (2006).
(23) I. Brondz, D. Ekeberg, D.S. Bell, A.R. Annino, J. Arild Hustad, R. Svendsen, V. Vlachos, P. Oakley, G.J. Langley, T. Mohini, and C-G. Amaury, F. Mikhalitsyn, J. Pharm. Biomed. Anal. 43, 937 (2007).
(24) D.R. Gere, Science 21, 253 (1983).
(25) J.F Parcher and J.R Strubinger, J. Chromatogr. 479, 251 (1989).
(26) J.R. Strubinger, H. Song, and J. F. Parcher, Anal. Chem., 63(2), 104 (1991).
(27) M. R. Emmett, S. Kazazic, A. G. Marshall, W. Chen, S.D.-H. Shi, B. Bolan, and M. J. Greig, Anal. Chem. 78, 7058 (2006).
(28) J. Kirkland, Anal. Chem. 41, 218 (1969).
(29) J. Kirkland, Anal. Chem. 64, 1239 (1992).
(30) J. Kirkland, F. Truszkowski, C. Dilks, and G. Engel Jr., J. Chromatogr., A 890, 3 (2000).
(31) J. Knox and J. Vasvari, J. Chromatogr. 83, 181 (1973).
(32) X. Wang, W. Barber, and P. Carr, J. Chromatogr., A 1107, 139 (2006)
(33) F. Gritti, I. Leonardisa, D. Shock, P. Stevenson, A. Shalliker, and G. Guiochon, J. Chromatogr., A 1217, 1589 (2010).
(34) P.A. Mourier, M.H. Caude, and R.H. Rosset, Chromatographia 23, 21 (1987).
(35) P.J. Schoenmakers and L.G.M. Uunk, Chromatographia 24, 51 (1987).
(36) P.J. Schoenmakers, in Supercritical Fluid Chromatography, R.S. Smith Ed. (Royal Society of Chemistry, London, UK 1988), Ch. 4.
(37) D.P. Poe and D.E. Martire, J. Chromatogr. 517, 3 (1990).
(38) H.G Janssen, H.M.J. Snijders, J.A.Rijks, C.A. Cramers, and P.J. Schoenmakers, J. High Resolut. Chromatogr. 14, 438 (1991).
(39) T.A. Berger and W.H. Wilson, Anal. Chem. 65, 1451 (1993).
(40) P. Sandra, A. Kot, and F. David, Chem. Today 9, 33 (1994).
(41) K.W. Phinney, L.C. Sander, and S.A. Wise, Anal. Chem. 70(11), 2331 (1998).
(42) C. Brunelli, Y. Zhao, M.-H. Brown, and P. Sandra, J. Chromatogr., A 1185, 263, (2008).
(43) D.M. Heaton, K.D. Bartle, A.A. Clifford, M.S. Klee, and T.A. Berger, Anal. Chem. 66, 4253 (1994).
(44) C. West and E. Lessiller, J. Chromatogr., A 1115, 233 (2006).
(45) C. West and E. Lessiller, J. Chromatogr., A 1110, 200 (2006).
(46) C. West and E. Lesellier, J. Chromatogr., A 1169, 205 (2007).
(47) C.West, J. Ogden, and E. Lesellier, J. Chromatogr., A 1216, 5600 (2009).
(48) E. Lesellier, J. Chromatogr., A 1216, 1881 (2009).
(49) C. West and E. Lesellier, J. Chromatogr., A 1203, 105 (2008).
(50) H. Bui, T. Masquelin, T. Perun, T. Castle, J. Dage, and M.-S. Kuo, J. Chromatogr., A 1206, 186 (2008).
(51) A.Cazenave-Gassiot, R. Boughtflower, J. Caldwell, R. Coxhead, L. Hitzel, S. Lane, P. Oakley, C. Holyoak, F. Pullen, and G.J. Langley, J. Chromatogr., A 1189, 254 (2008).
(52) C. White, J. Chromatogr., A 1074, 163 (2005).
(53) M. Maftouh, C. Granier-Loyaux, E. Chavana, J. Marini, A. Pradines, Y. Vander Heyden, and C. Picard, J. Chromatogr., A 1088, 67 (2005).
(54) Y. Zhang, W. Watts, L. Nogle, and O. McConnell, J. Chromatog. A 1049, 75 (2004).
(55) L. Miller and M.Potter, J. Chromatogr., B 875, 230 (2008).
(56) Z. Pirzada, M. Personick, M. Biba, X. Gong, L. Zhou, W. Schafer, C. Roussel, and C.J. Welch, J. Chromatogr., A 1217, 1134 (2010).
(57) D. Mangelings and Y. Vander Heyden, J. Sep. Sci. 31, 1252 (2008).

Perspectives in Hydrophobic Interaction Temperature- Responsive Liquid Chromatography (TRLC)
TRLC can obtain separations similar to those of reversed-phase LC while using only water as the mobile phase.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





