Recent Developments in High-Abundance Protein Removal Techniques
LCGC North America
In the last year, two new protein depletion products have been introduced, and these will be the focus of this month's installment of "Directions in Discovery."
The utility of protein biomarkers as indicators of disease states or as measures of response to therapy is well established. However, the number of validated biomarkers is limited, and despite immense efforts to discover novel biomarkers over the last decade, the success rate has been alarmingly low. Biomarker discovery studies most often employ serum and plasma because samples are easy to obtain. Moreover, blood is in contact with the entire organism and should contain tissue leakage proteins, which would be indicative of disease-induced damage. However, the ability to discover low-abundance biomarker proteins is hampered by the dominance of high-abundance proteins (HAPs). The serum and plasma proteomes span a concentration range of 11 orders of magnitude, and the 20 most abundant proteins account for 97–99% of the total protein mass (1). Detecting a candidate biomarker at picogram-per-milliliter concentration against a background of housekeeping proteins in milligram-per-milliliter concentrations is a formidable analytical challenge. To successfully identify these low-abundance proteins, one must find a way to "climb down the Anderson ladder" by first eliminating high- and medium-abundance proteins (Figure 1).

Tim Wehr
Conventional approaches to simplifying the serum or plasma proteome target specific members of the class of HAPs because they are few in number but account for the majority of proteome mass. Albumin, which makes up 50–70% of the proteome, can be depleted by pseudo-affinity techniques such as Cibacron Blue chromatography. Immunoglobulin G, which makes up 8–26% of the proteome, can be depleted by affinity chromatography on protein A or protein G columns. Unfortunately, removal of one or both of these proteins still leaves an unmanageable analytical problem. Immunoaffinity chromatography has emerged as the most viable technique for removal of multiple HAPs, and several commercial products for immunoaffinity depletion are now available. These products were reviewed by Zolotarjova, Mrozinski, and Majors in the February 2007 installment of "Sample Prep Perspectives" (2) and will be described only briefly here. The focus of this report will be products that have appeared since the previous review.
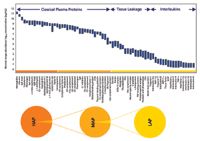
Figure 1: Dynamic range of human plasma.
Single-Step Immunodepletion Products
In the last three years, immunodepletion materials for HAP removal from human serum and plasma have been introduced by Agilent Technologies (Santa Clara, California), Sigma Aldrich (St. Louis, Missouri), and Genway Biotech (San Diego, California). All of these are designed for single-step depletion, and, depending upon the product, can remove as many as 20 HAPs. These products can show cross-reactivity with serum or plasma from other mammals.
Multiple affinity removal system: The multiple affinity removal system (MARS) from Agilent Technologies was one of the first immunoaffinity depletion systems to be commercialized. It consists of a mixture of po clonal antibodies to six human serum HAPs (serum albumin, IgG, α-1-antitrypsin, haptoglobin, and IgA) attached to polymeric beads. Antibodies are attached through their Fc regions to the polymeric support; this linkage provides easy access of proteins to the affinity binding sites. The MARS beads are available in both spin column and HPLC column formats. Depletion efficiencies of >99% have been reported, and average recovery of spiked tumor markers was 78% (3). Later additions to the product line include a Human-7 column able to deplete the original six HAPs plus fibrinogen, and a Mouse-3 column for depletion of murine albumin, immunoglobulin, and transferrin.
ProteoPrep 20 plasma immunodepletion kit: The ProteoPrep 20 immunodepletion technology from Sigma-Aldrich employs a mixture of antibodies to 20 human plasma HAPs (Table I). The antibodies include polyclonal IgGs and single-chain antibodies conjugated to a support via linkages designed to reduce non-specific binding. This material is available in spin column and high performance liquid chromatography (HPLC) column formats. It can remove 98% of the plasma protein mass in a single passage of sample through the bed and >99% in a second pass. The average depletion efficiency for the 20 proteins is 99.3% (4,5).

Table I: High abundance proteins removed from human plasma by the ProteoPrep 20 plasma immunodepletion technology
Seppro IgY-based immunodepletion products: Genway Biotech has developed a family of immunodepletion products based upon avian yolk (IgY) antibodies (6). These are prepared by introducing a purified human HAP as antigen into laying hens. The corresponding IgY antibody is secreted into the egg yolk, and can be harvested easily and purified by affinity capture on columns carrying the protein antigen. Each purified IgY is conjugated to bisacrylamide–azlactone copolymer microbeads. Coupling is via the oligosaccharide moieties on the IgY heavy chain domains. The immunodepletion reagent is prepared by blending individual antiHAP IgY beads to form mixtures for depletion of 6, 12, or 14 HAPs (Table II). The immunodepletion products are available in bulk and in spin column and HPLC column formats. Use of IgY antibodies offers several advantages over IgG-based immunodepletion systems. First, the IgY antibodies exhibit high affinity for HAPs. Second, the Fc regions of IgY antibodies do not bind human proteins. This is in contrast to IgG Fc regions, which are known to bind complement proteins, rheumatoid arthritis factors, human anti-mouse antibodies, and Fc binding proteins. This lack of cross-reactivity makes IgY antibodies more target-specific. A third advantage of IgY antibodies is the ease with which all the target proteins can be stripped from their cognate IgYs, allowing the beads to be recycled multiple times. Finally, due to the evolutionary distance between birds and mammals, antihuman IgYs exhibit a broader antigen-binding host range than IgGs. This allows the antihuman IgYs to be used for depletion of other mammalian proteomes such as mouse, rat, or goat.

Table II: High abundance proteins removed from human plasma by the Seppro single-step immunodepletion products
ProteoSpin abundant serum protein depletion kit: The Norgen Biotek Corp. (Thorold, Ontario, Canada) has taken a different approach to HAP depletion. The company's ProteoSpin column is designed to remove HAPs using an ion-exchange mechanism, in contrast to the antibody-based products described previously. Using proprietary activation, wash, elution, and neutralizer solutions, the spin column depletes albumin, α-antitrypsin, transferrin, and haptoglobin from human serum or plasma. The ion-exchange approach is cost-effective and can be used for HAP depletion from samples of different animal origin. Both salts and HAPs pass through the column in the flow-through, and bound proteins are recovered in the elution step.
Multistep Immunodepletion Products
One concern about the use of a single-step procedure to remove the HAPs from serum or plasma is that medium-abundance proteins (MAPs) remaining in the flow-through can block access to the low-abundance proteins (LAPs) that are most relevant to discovery of novel biomarkers. Genway has addressed this problem by coupling its IgY-12 and -14 HAP removal system with a second immunodepletion column that removes MAPs (7). This column, the SuperMix, is prepared by immunizing chickens with the flow-through from the IgY-12 HAP depletion column. The anti-MAP IgY antibodies are purified from egg yolks using affinity column chromatography and then are conjugated to microbeads. For plasma protein depletion, a sample is first passed through an IgY-12 or IgY-14 column to remove HAPs, and in the second step, the flow-through is introduced into the SuperMix column. The SuperMix column removes over 200 MAPs, and the SuperMix flow-through is enriched for LAPs (Table III and Figure 2).
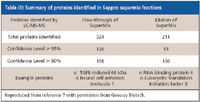
Table III: Summary of proteins identified in Seppro supermix fractions
Recently, both the Agilent 7-antibody and the Genway IgY-14 one-step antibody methods were compared with the two-step IgY-12 + SuperMix for depletion of human plasma proteins (8). The two-step procedure enabled detection of plasma proteins at lower concentration than either of the one-step procedures.
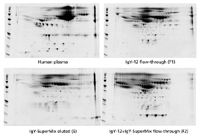
Figure 2: Two-dimensional gel electrophoresis analysis of unfractionated human plasma proteins, proteins in IgY-12 flow-through, proteins bound and eluted from the SuperMix column, and proteins in the SuperMix flow-through. Reproduced with permission from Genway Biotech.
LAPs identified by LC–MS following two-step depletion included insulin-like growth factor-binding proteins, L-selectin, fibrillin-1, galectin-3-binding protein, cartilage oligomeric matrix protein, and mannose-binding protein C.
Protein Depletion Using Peptide Affinity
Immunodepletion using antibody-based products has two limitations. First, the sample can be diluted during the elution step. Second, ever-deeper mining of the proteome requires an ever-expanding set of immunodepletion products. An alternative approach uses a combinatorial library of hexapeptides bound to a chromatography support. This approach, which forms the basis of the ProteoMiner technology from Bio-Rad Laboratories (Hercules, California), overcomes most of the disadvantages of immunodepletion while still effectively depleting HAPs (9). Using "split-couple-recombine" combinatorial systhesis, a library of hexapeptides bound to macroporous 65-μm-diameter polymethacrylate microbeads is produced, with each bead carrying a unique peptide sequence. The sequential coupling of the 20 protein amino acids results in a library of 206 unique beads. These are packed in spin columns with a 100-μL bed volume. In contrast to antibody-based immunodepletion products, which typically have a capacity of 100 μL or less, sample volumes of 1 mL and more can be applied to the ProteoMiner beads. Because there are a limited number of binding sites for each protein, HAPs quickly reach the bead capacity, and excess HAPs are passed through the column. After sample introduction and washing, bound proteins are eluted from the column with a small volume (≤300 μL) of elution buffer (5% acetic acid, 8 M urea, 2% CHAPS). The net result is depletion of HAPs accompanied by at least a threefold increase in the concentration of LAPs. Using human plasma, quantitative recovery (99.6%) of applied protein in the flow-through, wash, and eluted fractions was obtained, while almost 96% of the applied protein was removed in the flow-through and wash steps. Two-dimensional gel electrophoresis analysis of serum and plasma samples treated with ProteoMiner beads demonstrated depletion of HAPs and increased spot intensity and resolution for LAPs (Figure 3). Moreover, the treated samples exhibited unique spots, and an increase in spot count of 32% for serum and 52% for plasma. Protein identification by in-gel tryptic digestion followed by LC-tandem MS produced higher-confidence identification scores for LAP spots in the treated samples. The LC–MS analysis of unique spots from the treated samples identified them as proteins known to exist in low to medium abundance in plasma.

Figure 3: Two-dimensional gel electrophoresis analysis of plasma and serum samples treated with ProteoMiner beads. For both untreated and treated samples, 100 μg of protein was analyzed using a pH 5-8 IPG strip in the isoelectric focusing dimension. Reproduced from reference 9 with permission from Bio-Rad Laboratories.
Conclusions
A number of commercial protein-depletion products are available currently for simplifying the task of deep-mining the proteome in the search for protein biomarkers. Most of these are single-step antibody-based immunodepletion products for removal of as many as 14 of the highest-abundance proteins in human serum or plasma. The recent introduction of a multistep immunodepletion product and a peptide affinity-based technology have the potential for reaching that segment of the proteome where novel biomarkers are hoped to reside.
Tim Wehr
"Directions in Discovery" editor Tim Wehr is staff scientist at Bio-Rad Laboratories, Hercules, California. Direct correspondence about this column to Direct correspondence about this column to "Directions in Discovery," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com
References
(1) N.L Anderson and N.G. Anderson, Mol. Cell. Proteomics 1, 845 (2002).
(2) N. Zolotarjova, P. Mrozinski, and R.E. Majors, LCGC 25(2), 118 (2007).
(3) J. Brand, T. Haslberger, W. Zolg, G. Pestlin, and S. Palme, Proteomics 6, 3236 (2006).
(4) M. Schuchard, C. Melm, A. Crawford, H. Chapman, S. Cockrill, K. Ray, R. Mehigh, D. Chen, and G. Scott, presented at the NCI Proteomics Technologies Reagents Resource Workshop, Dec. 12–13, 2005.
(5) C. Melm, M. Schuchard, A. Crawford, H. Chapman, C. Ngowe, K.Ray, and D. Chen, presented at ABRF 2007.
(6) L. Huang, G. Harvie, J.S. Feitelson, K. Gramatikoff, D.A. Herold, D.L. Allen, R. Amunngama, R.A. Hagler, M.R. Pisano, W.-W. Zhang, and X. Fang, Proteomics 5, 3314 (2005).
(7) X. Fang, L. Huang, D. Hinerfeld, S. Tam, P. Gagné, G.G. Poirier, C. Kusumoto, K. Obata, S. Sikora, and W.-W. Zhang, Abstract 585, presented at the 55th ASMS Conference on Mass Spectrometry and Allied Topics, June 2007.
(8) H. Lin, T.A. Shaler, J. Wang, M. Chen, E. Price, J. Kothule, S. Chen, and C.H. Becker, Abstract 1228, presented at the 55th ASMS Conference on Mass Spectrometry and Allied Topics, June 2007.
(9) A. Paulus, S. Freeby, K. Academia, V. Thulasiraman, T. Wehr, N. Liu, S. Roth, and K. Smith, Bio-Rad Technical Note 5632 (2007).


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









