Real-Time Monitoring of Volatile Organic Compounds in Ambient Air Using Direct-Injection Mass Spectrometry
Exposure to volatile organic compounds (VOCs) at low parts per billion (ppb) concentrations (by volume, ppbV) in ambient air is increasingly being recognized as a health risk. Furthermore, VOCs contribute to the formation of photochemical smog and particulate matter, which are hazardous and negatively impact the environment. Although most urban VOCs are anthropogenic in origin, some are produced by living organisms, especially trees. Gas chromatography (GC) is conventionally applied to VOC analysis of air, but it generally has a time resolution of tens of minutes and detection at low ppbV levels, frequently requiring sample preconcentration (such as using thermal desorption tubes (TDTs) or canister sampling with cryotrapping prior to analysis). Newer direct-injection mass spectrometry (DIMS) techniques can address the challenge of time resolution (seconds to tens of seconds) and eliminate the need for sampling via TDTs or canisters while still providing sufficient specificity. In this article, the results of a DIMS air monitoring campaign in Auckland, New Zealand, are described. Real-time selected ion flow tube mass spectrometry (SIFT–MS) measurements were obtained at three sites over a period of several weeks each: (i) adjacent to a freeway, (ii) in a residential area, and (iii) at a rural location. There were 13 compounds that were targeted in the study, covering various sources: fugitive emissions and incomplete combustion (benzene, toluene, and ethylbenzene, along with the xylene isomers, styrene, and ethanol), incomplete combustion (1,3-butadiene, formaldehyde, acetaldehyde, acrolein, benzaldehyde, and acetone), and biogenic origin (isoprene and monoterpenes (total of all isomers). At each site, SIFT–MS provided effective on-site analysis to sub-ppbV levels with better than 1-min time resolution.
Volatile organic compounds (VOCs) are chemically diverse but all have significant vapor pressure at room temperature. In addition to causing negative health effects, VOCs are significant contributors to photochemical smog and the formation of particulate matter, impacting both the climate and human health (1).
Analyzing VOCs is most commonly conducted using “gold standard” gas chromatography (GC) techniques. However, analysis at ambient concentrations using GC requires sample preconcentration, which means off-line analysis is necessary. Furthermore, chromatographic separation typically limits time resolution to tens of minutes, which is problematic for ambient air because it is a dynamic matrix subject to wind shifts and other short timescale disturbances.
Adoption of direct-injection mass spectrometry (DIMS) techniques, such as proton transfer reaction mass spectrometry (PTR–MS) and selected ion flow tube mass spectrometry (SIFT–MS), can address these limitations through direct, continuous analysis of ambient air (2). DIMS techniques usually employ soft chemical ionization, which enables analysis to occur in the presence of nitrogen, oxygen, and moisture because these species are not ionized significantly. The challenge for DIMS techniques is assuring specificity because soft ionization produces fragmentation patterns that are less unique than those obtained using electron ionization (EI); also, there is no orthogonal dimension as there is for GC (retention time, tR). Although not truly orthogonal, the use of multiple reagent ions with different gas-phase ionization mechanisms is an effective way to increase specificity in DIMS techniques. In contrast to PTR–MS (3), which can only change reagent ion on a timescale of seconds to minutes, and hence has sought to address specificity concerns by increasing mass resolution through time-of-flight mass spectrometry (TOF–MS), SIFT–MS has fast-switching capability for its reagent ions (see “Methods”) and usually provides good speciation when coupled with quadrupole MS (QMS) (4).
Although QMS has advantages over TOF–MS for stable, remote ambient air monitoring, conventional SIFT–MS has an economic limitations, unlike PTR–MS. The flow-based approach to sample ionization used in SIFT–MS requires carrier gas (conventionally expensive helium), whereas the PTR–MS technique successfully ionizes VOCs in undiluted air by utilizing a drift tube (3). To address this issue, laboratory experimentation using nitrogen carrier gas was undertaken because ultrahigh-purity nitrogen is economical both as a bottled gas or when it is continuously generated. Initial laboratory results indicated excellent comparability for nitrogen and helium carrier gases (5), so a field campaign was conducted to evaluate the suitability of ambient monitoring when using nitrogen carrier gas. The study, which is described in this article, focused primarily on compounds of biogenic origin, pollutants from motor vehicles (which includes the aromatics from the United States Environmental Protection Agency’s Compendium methods TO-14A (6) and TO-15 (7) compendium methods), and the volatile chemical products (VCPs) acetone and ethanol. Although the campaign occurred in 2015, it is perhaps more relevant now than it was at the time it was conducted because of the increased awareness of VCPs as major VOC sources in the urban atmosphere (1,8).
Methods
SIFT–MS Instrumentation and Sampling Configuration
SIFT–MS is a DIMS technique that provides real-time analysis of VOCs in air to trace concentrations (9) and has been described in detail in the literature (10,11). Data obtained by SIFT–MS instruments compare well with accepted gas chromatography–mass spectrometry (GC–MS) methods for VOC analysis in applications as diverse as ambient air sampling from canisters (12) and plasma headspace (13).
The SIFT–MS instrument used here (a Voice200ultra model from Syft Technologies) was operated on nitrogen carrier gas (grade ultra-high purity from Southern Gas Services) and utilized the three standard SIFT–MS positively charged reagent ions: H3O+, NO+, and O2+ (11). Sample delivery to the instrument utilized a prototype three-port inlet, which enabled con- nection to the ambient air sampling line, blank gas (nitrogen), and a gas standard used to check instrument performance (the so-called “Syft Standard” from Air Liquide). The instrument sampled continuously at a flow rate of 25 standard cubic centimeters per minute (sccm) from the selected sample port via a calibrated capillary that controlled introduction from atmospheric pressure into the instrument flow tube (held at 400 mTorr for nitrogen, 600 mTorr for helium systems). Residence times in the blank and standard lines were minimal because of the short lengths of 1⁄4-in o.d. tubing used. The residence time in the ambient line (25 mm o.d.; outlet to atmosphere 4 m above ground level) was minimized by pumping a fast flow of ambient air past the instrument’s capillary inlet using an oil-less diaphragm pump (DOA model from GAST Manufacturing); for this study, a flow-past sampling configuration was used.
Batches were configured in the instrument software to enable the regular collection of blank data (approximately once every 2 hr at the first site and 5 hr at the other sites) and a daily run of the performance check standard. Otherwise, ambient data was collected pseudo-continuously using a 5.5-min analysis repeatedly because of the instrument computer taking several seconds to save the run data and reset the ion source pressure. Blank data were subtracted during data post-processing. Ambient and blank methods used a maximum ion dwell time of 1 s (the instrument stays at the selected mass-to-charge ratio (m/z) for 1 s unless 10,000 ions are counted first).
Unattended 24/7 operation of the instrument was monitored via a cellular data connection. Through this cellular collection, analytical data were transferred every few days.
Analytical Approach and Method Development
The primary aim of this study was to investigate the suitability of the SIFT–MS technique for unattended, continuous monitoring of ambient VOC concentrations for durations of several weeks when operating on nitrogen carrier gas. Therefore, quantitation was conducted from the in-built chemical ionization library (14) rather than via calibration because the accuracy obtained from the library was adequate for this purpose based on previous published work (12). Here, repeatable measurement is most important and has been demonstrated elsewhere (15).
Table I summarizes the 13 VOCs targeted in this study and the reagent ion-product ion pairs utilized to detect and quantify them. Cited references for the SIFT–MS detection parameters are for conventional helium carrier gas. However, branching ratios were re-confirmed in nitrogen carrier gas and minor adjustments were made in a minority of cases; the laboratory studies confirmed that no new product ions were formed. In the context of the present study, formal determinations of the limits of quantitation (LOQ) for each compound were not made. Conservative estimates are provided in Table I based on the steady baseline concentrations reported at the rural monitoring site.
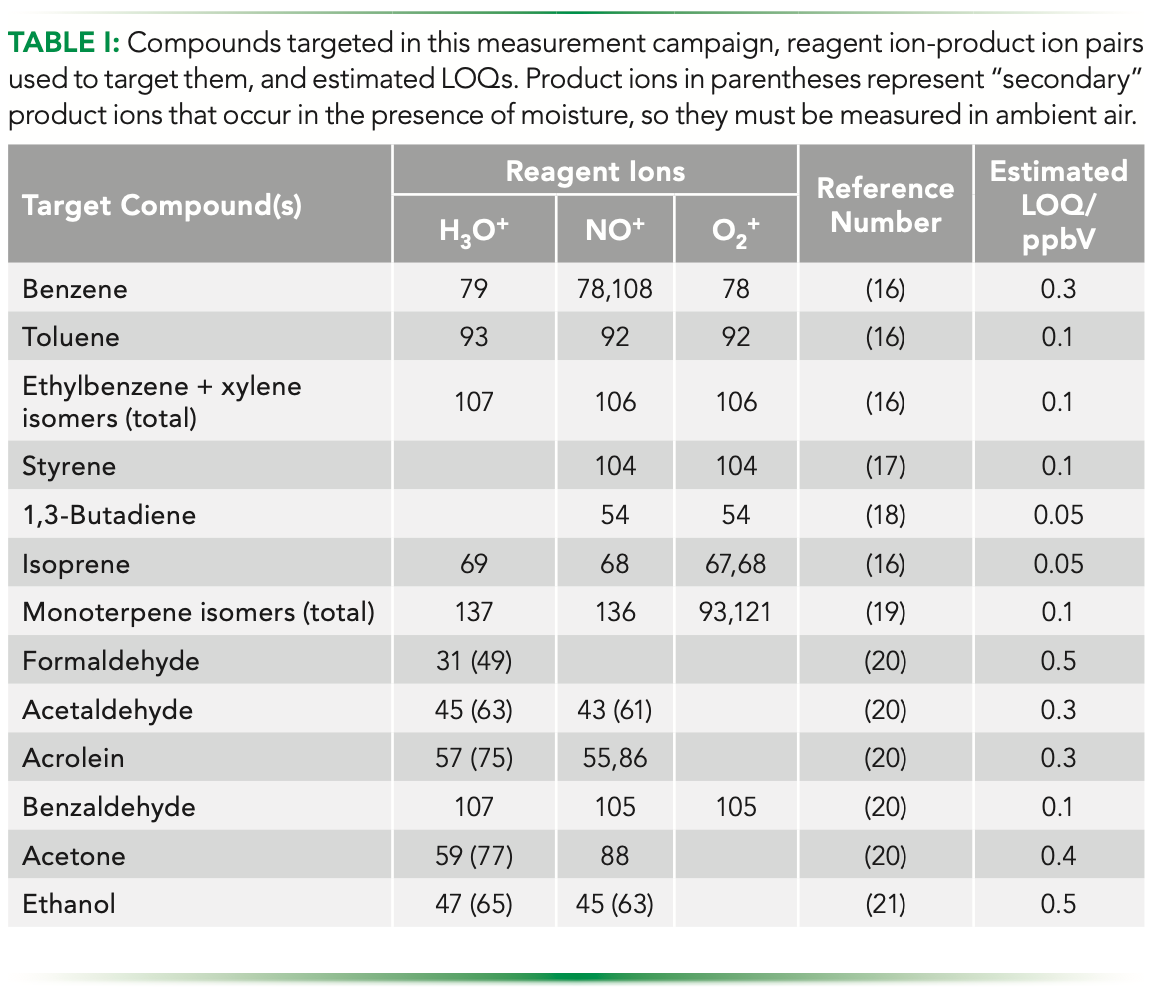
In this campaign, a time resolution of ca. 1 min was sufficient in maximizing specificity two or more product ions were used to independently quantify analytes (only formaldehyde was targeted using a single product ion because it has no other usable reactions with the positive reagent ions). The rapid, quadrupole-facilitated switching of reagent ions in SIFT–MS instruments enabled product ions for an analyte to be selected across multiple reagent ions in the same, continuous analysis, providing enhanced real-time specificity. The reagent ions usually react via different gas-phase ionization mechanisms, and for a given reagent ion, the relevant mechanisms are determined by the analyte functional groups. When these factors are taken together, the availability of three rapidly switchable reagent ions often yields one or more unique product ion m/z (z = 1 for SIFT–MS ionization) from which quantitation can be achieved.
Figure 1 illustrates the cycling of the reagent ion signal as a function of time for the instrument method used in this study. A data point for each product ion is recorded on every cycle through the reagent ions, and the concentrations calculated for each product ion are automatically compared with the lowest being reported (or an averaged value if several product ions yield concentrations within 20% of the lowest value). Higher values indicate that a product ion is subject to interference.
FIGURE 1: Reagent ions: H3O+ (m/z 19), NO+ (30), and O2+ (32) are cycled rapidly in the SIFT–MS method, enabling the most sensitive and specific product ions to be used to target each compound while also providing interference rejection.
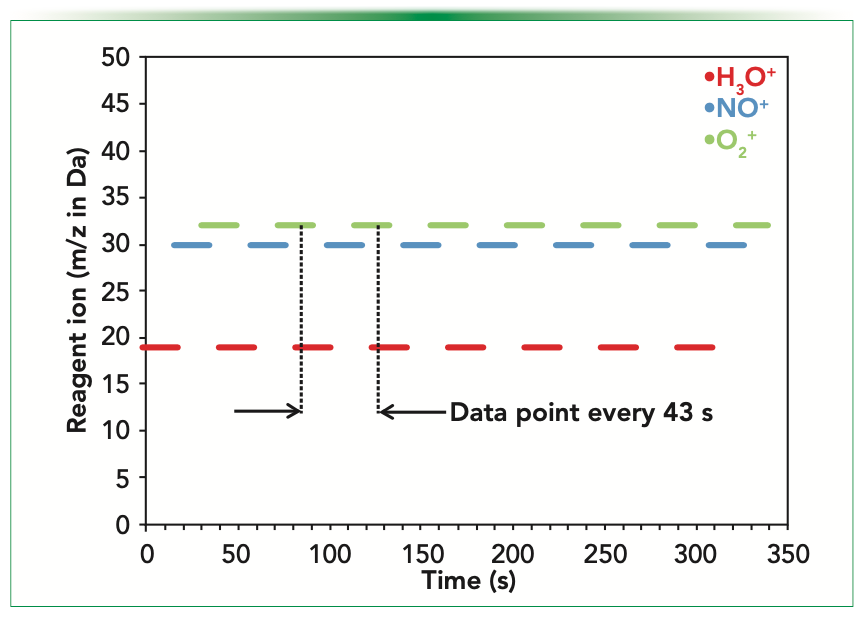
Monitoring Sites
The ambient air monitoring campaign was conducted in Auckland, New Zealand (population 1.7 million in 2021), in collaboration with the local authority, the Auckland Council. The SIFT–MS instrument was located for several weeks each at three air monitoring stations operated by Watercare Services on behalf of the Auckland Council (Figure 2):
1. A suburban residential site in the suburb of Glen Eden approximately 8 miles (13 km) from Auckland’s central business district (CBD).
2. A rural site (Patumahoe) approximately 22 miles (35 km) south of the Auckland CBD and 10 miles (16 km) inland from the Tasman Sea.
3. A site in Takapuna adjacent to the northern freeway approximately 2 miles (3 km) north of Auckland harbor, and 5 miles (8 km) north of the CBD.
The selected sites range from roadside to near ambient background concentrations, which provide a good range over how to assess the SIFT–MS technique.
FIGURE 2: Ambient air monitoring sites in Auckland, New Zealand, where the SIFT–MS instrument was located during the campaign. The inset indicates the location of the Auckland region in New Zealand.
Results and Discussion
In this section, the results obtained in the ambient monitoring campaign are presented and discussed site-by-site.
Residential Suburban Site:
February 7 to 23 (Late Summer)
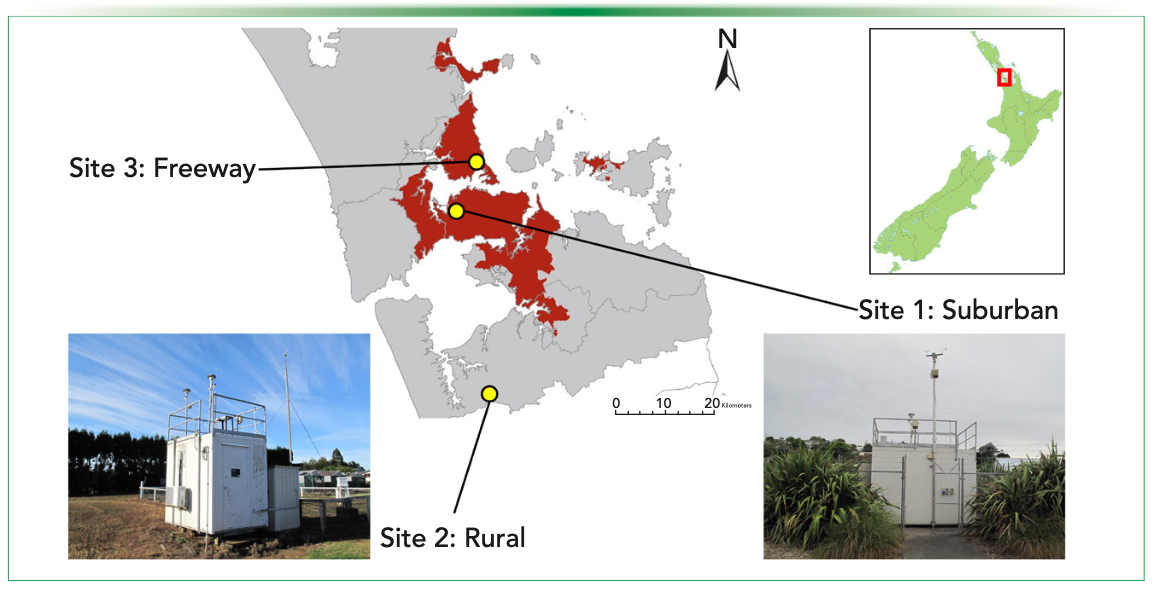
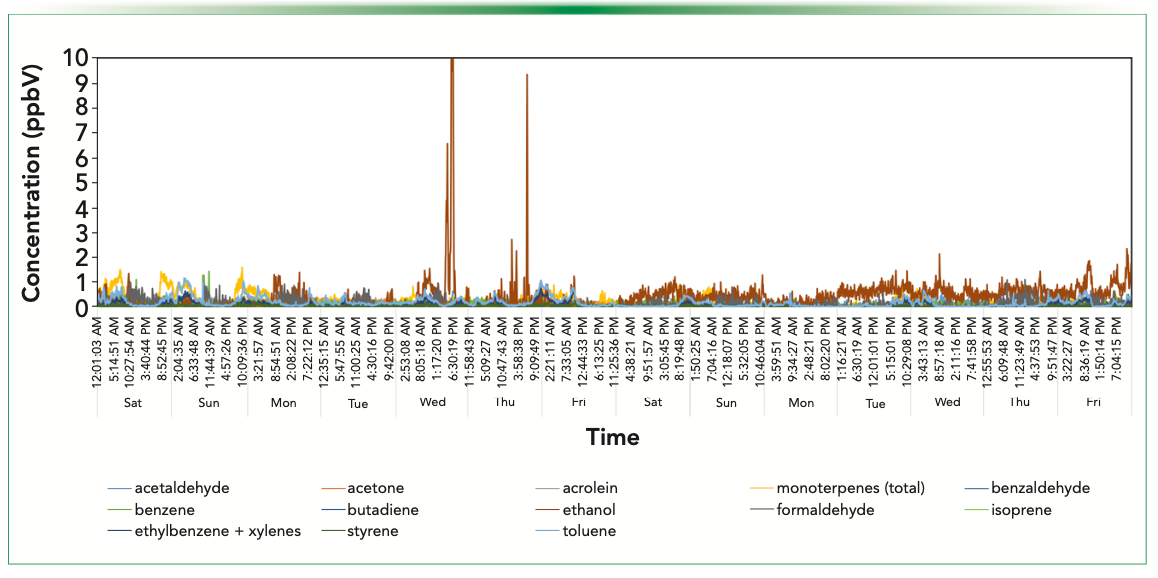
Figure 3 shows ambient monitoring data acquired in the quiet, largely residential area of Glen Eden. This site lies in a shallow basin surrounded by low hills and has significant vegetation to the west. Nevertheless, it is still subject to breezy conditions that characterize Auckland weather. The monitoring site has no nearby arterial roads, and the local roads carry very low volumes of traffic. Generally, compound concentrations correlate inversely with wind speed; that is, a lower wind speed means that higher val- ues are measured. However, there is one somewhat unusual feature of the data in that several compounds that are ordinarily associated with motor vehicle emissions (most notably toluene) occur at higher concentrations in the middle of the night. The monitoring station is located near the boundary of a multi-use sports ground constructed on an historic landfill. It is probable that the source of toluene is fugitive emission from the historic landfill (since traffic cannot be the cause), which lingers in the vicinity longer during calm night conditions.
FIGURE 3: Windspeed and SIFT–MS concentration data for the residential site in Glen Eden, Auckland, New Zealand.
Ethanol is moderately abundant at certain times but does not correlate with toluene. Peak concentrations tend to occur mid-evening and are largest on Friday, Saturday, and Sunday evenings. This behavior occurs because of the consumption of alcoholic beverages at the nearby sports club or houses immediately behind the monitoring station or from VCP emissions from neighboring homes. In the United Kingdom, VCPs are now a significant contributor (63%) to atmospheric VOCs loads, with ethanol being the most abundant (1).
Rural Site: April 4 to 17 (mid-Fall)
During the second portion of the campaign, the SIFT–MS instrument was located at a rural monitoring station. Unfortunately, the local authority does not collect wind data at this site. VOC concentrations (Figure 4) were lower than the other sites because prevailing westerly wind conditions carry air masses across the Tasman Sea and then through approximately 10 miles of farmland prior to analysis. It is possible that ethanol (peak concentration at 15 ppbV) and the monoterpenes contributed from vegetation, agricultural activities, or both. When easterly wind conditions arise, VOCs of anthropogenic origin, such as formaldehyde and toluene, could be carried to the site from Pukekohe township (2021 population of 27,000) that lies just 3 miles (5 km) east of Patumahoe. However, without wind data, this theory remains speculative. Nevertheless, it can be concluded from the results obtained at this monitoring site that SIFT–MS has the potential to analyze background VOC levels to well below 1 ppbV.
FIGURE 4: SIFT–MS concentration data for the rural background site in Patumahoe, Auckland. Note the different concentration scale compared to Figures 3 and 5.
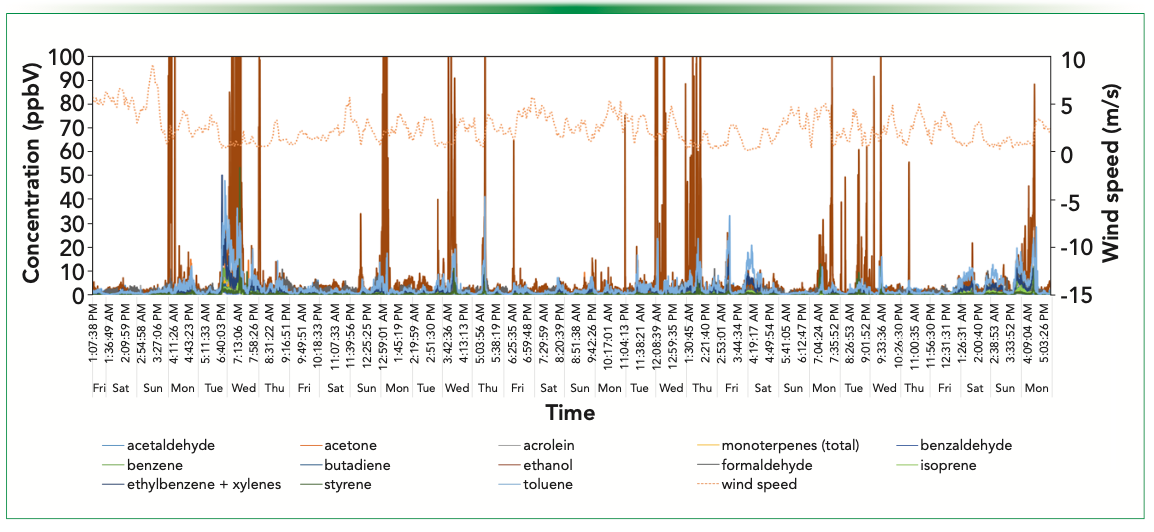
Freeway Site: June 12 to July 13 (early Winter)
The final air monitoring site in the campaign was located approximately 200 ft (60 m) east of the major north-south freeway in Takapuna, which lies on the northern side of Auckland harbor. The results obtained are shown in Figure 5. Ethanol is the dominant volatile at this site, with several periods registering concentrations greater than 400 ppbV (on Monday June 15th and Monday June 22nd). However, despite the proximity of the site to the freeway, it is very unlikely that the ethanol source is vehicular emissions—even though concentrations are highest when there is a light westerly breeze—because only one smaller New Zealand gasoline retailer offers ethanol blends. Toluene is the next most abundant VOC measured, and it may arise from both incomplete fuel combustion and volatile chemical products (8) because it is frequently observed when traffic volumes are low and wind conditions are light. Styrene is observed at times, likely arising from light industry in the vicinity of the monitoring station. Despite the site being adjacent to a freeway, vehicular VOC emissions cannot be distinguished from the VCP contributions.
FIGURE 5: Windspeed and SIFT–MS concentration data for the freeway site in Takapuna, Auckland.
Conclusions
The results presented here demonstrate that a SIFT–MS instrument operating on nitrogen carrier gas can be utilized for unattended, continuous monitoring of VOCs at ambient concentrations. Nitrogen is an economical solution for continuous monitoring because a standard gas cylinder (8 m3) costs less than helium and lasts over three weeks for continuous analysis, whereas the equivalent volume of the expensive, conventional helium carrier gas lasts less than 10 days. Longer periods of unattended operation can be achieved using multi-cylinder supplies or high-purity nitrogen generators, which are readily available commercially.
The SIFT-MS measurements reported in this study provided concentration data for 13 analytes with a time resolution of better than 1 min, with the potential for additional analytes to be added while retaining that time resolution. This ability is a clear benefit in using DIMS compared to conventional GC analysis because variations in concentrations as a function of wind speed and direction can be observed. Moreover, by measuring both hydrocarbons and oxygenated VOCs in a single analysis, SIFT–MS can address the need that is identified by Lewis and others (1) for measuring oxygenated VOCs in the modern urban atmosphere in which the dominant atmospheric VOCs arise from VCPs rather than motor vehicles.
Finally, rapidly switchable reagent ions enabled the SIFT–MS technique to analyze all VOCs in the method with high specificity. For example, although H3O+ must be used for formaldehyde analysis, only having this reagent ion would mean that the benzaldehyde product ion could not be distinguished from the isobaric products ions (with unit m/z resolution provided by a QMS) of the xylenes and ethylbenzene. Therefore, multiple, switchable reagent ions are a beneficial feature for ambient air analysis.
Acknowledgments
The author is grateful to Mr. Nick Reid and Mr. Matt Hope at Auckland Council, and Mr. Stephen Sing at Watercare Services, all of whom supported this campaign and arranged access to monitoring sites. Special thanks to the following colleagues at Syft Technologies—Dr. Diandree Padayachee, Mr. John Gray and Ms. Jamie Buckland—who assisted with logistics, data collection, and processing.
References
(1) A.C. Lewis, J.R. Hopkins, D.C. Carslaw, J.F. Hamilton, B.S. Nelson, G. Stewart, et al, Philos. T. Roy. Soc. A 378, 2183 (2020). DOI: 10.1098/rsta.2019.0328, 2020.
(2) M.J. McEwan, in Ion Molecule Attachment Reactions: Mass Spectrometry, T. Fujii, Ed. (Springer, New York, NY, 2015), pp. 263–317.
(3) R.S. Blake, P.S. Monks, and A.E. Ellis, Chem. Rev. 109, 861–896 (2009). DOI: 10.1021/ cr800364q
(4) A.-S. Lehnert, E. Perreca, J. Gershenzon, G. Pohnert, and S.E. Trumbore, Front. Plant Sci. 11, 578204 (2021). DOI: 10.3389/ fpls.2020.578204
(5) V.S. Langford, J.D.C. Gray, R.G.A.R. Maclagan, D.B. Milligan, and M.J. McEwan, Int. J. Mass Spectrom. 377, 490–495 (2015). DOI: 10.1016/j.ijms.2014.04.001
(6) Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air: Method TO-14A (2nd ed.) (U.S. Environmental Protection Agency, Research Triangle Park, NC, EPA 600/625/R-96/010b, 1999).
(7) Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air: Method TO-15 (2nd ed.) (U.S. Environmental Protection Agency, Research Triangle Park, NC, EPA 600/625/R-96-010b, 1999).
(8) B.C. McDonald, J.A. De Gouw, J.B. Gilman, S.H. Jathar, A. Akherati, C.D. Cappa, et al, Science 359, 760–764 (2018). DOI: 10.1126/science.aaq0524
(9) B.J. Prince, D.B. Milligan, and M.J. McEwan, Rapid Commun. Mass Spectrom. 24, 1763–1769 (2010). DOI: 10.1002/rcm.4574
(10) P. Spanel and D. Smith, Med. Biol. Eng. Comput. 24, 409–419 (1996). DOI: 10.1007/ BF02523843
(11) D. Smith and P. Spanel, Mass Spec. Rev. 24, 661–700 (2005). DOI: 10.1002/mas.20033
(12) V.S. Langford, I.Graves, and M.J. McEwan, Rapid Commun. Mass Spectrom. 28, 10–18 (2014). DOI: 10.1002/rcm.6747
(13) C. Hastie, A. Thompson, M.J. Perkins, V.S. Langford, M. Eddleston, and N. Homer, ACS Omega 6(48), 32818–32822 (2021). DOI: 10.1021/acsomega.1c03827
(14) Syft Technologies Limited, Compound Library, LabSyft Software package version 1.4.4, 2014.
(15) M.J. Perkins and V.S. Langford, Rev. Sep. Sci. 3(2), e21003 (2021). DOI: 10.17145/rss.21.003
(16) P. Spanel and D. Smith, Int. J. Mass Spectrom. 181, 1–10 (1998). DOI: 10.1016/S1387-3806(98)14114-3
(17) Syft Technologies Ltd, Compound Library, 2004.
(18) Syft Technologies Ltd, Compound Library, 2003.
(19) T. Wang, P. Spanel, and D. Smith, Int. J. Mass Spectrom. 228, 117–126 (2003). DOI: 10.1016/S1387-3806(03)00271-9
(20) P. Spanel, Y. Ji, and D. Smith, Int. J. Mass Spectrom. Ion Proc. 165/166, 25–37 (1997). DOI: 10.1016/S0168-1176(97)00166-3
(21) P. Spanel and D. Smith, Int. J. Mass Spectrom. Ion. Proc. 167/168, 375–388 (1997). DOI: 10.1016/S0168-1176(97)00085-2
ABOUT THE AUTHOR
Vaughan S. Langford is a Principal Scientist at Syft Technologies Limited, in Christchurch, New Zealand. Direct correspondence to: Vaughan.Langford@syft.com.


New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









