Quick and Convenient Comparison of Curry Powders Using Direct Thermal Desorption with GC-MS Analysis
The Application Notebook
Herbs and spices are used in many food preparations, and identifying the differences between samples is of particular interest to manufacturers, both for ongoing quality control and to compare their products against competitors. However, the volatile organic compound (VOC) profiles of such samples often differ in the relative abundance of key components, and these differences can be difficult to assess by traditional methods such as solvent extraction, equilibrium headspace, or solid-phase microextraction (SPME).
Caroline Widdowson, Gareth Roberts, and David Barden, Markes International
Herbs and spices are used in many food preparations, and identifying the differences between samples is of particular interest to manufacturers, both for ongoing quality control and to compare their products against competitors. However, the volatile organic compound (VOC) profiles of such samples often differ in the relative abundance of key components, and these differences can be difficult to assess by traditional methods such as solvent extraction, equilibrium headspace, or solid-phase microextraction (SPME).
In this application note we show the value of direct thermal desorption (TD) with analysis by gas chromatography-mass spectrometry (GC-MS) for assessing aroma profiles from small samples of curry powder.
Thermal Desorption
TD uses heat and a flow of inert gas to desorb VOCs and semiâvolatile organic compounds (SVOCs) from sorbents or sample materials. Extracted vapours are swept onto an electrically-cooled focusing trap, which is then rapidly heated to inject them into a gas chromatograph (GC).
TD offers many advantages over conventional solvent-based sample preparation methods such as liquid extraction. These include wider analyte range (from acetylene to n-C44 and reactive species on one platform), quantitative re-collection of split flows for repeat analysis and simple method validation, and enhanced sensitivity.
In this study, the TD-100™ automated cryogen-free thermal desorber from Markes International was employed, which has capacity for 100 industry-standard tubes.
Direct Desorption
Of the numerous TD-compatible sampling procedures, direct thermal desorption is the most straightforward and cost-effective for small quantities of relatively homogeneous, finely-divided materials - for example, therapeutic drugs, packaging materials, resins, spices, ointments/creams, polymers, water-based paints, and edible fats.
The material is simply weighed into an empty 3½-inch × ¼-inch TD tube, and heated directly within a thermal desorption instrument, followed by direct injection into the GC system. In this way, sample preparation is essentially reduced to zero, and the associated risk of introducing errors is eliminated.
Analysis of Curry powder
To illustrate the usefulness of direct desorption, Figure 1 shows the results obtained by direct desorption and TD-GC-MS analysis of two brands of curry powder. The range of analytes is very similar, but there are substantial differences in relative abundance. In particular, the cheaper brand (top) shows much higher quantities of linalool (#11), camphor (#12), and estragole (#13) compared to a midârange brand (bottom), but lower concentrations of cuminaldehyde (#14) and caryophyllene (#19).
Figure 1: Direct desorption of two brands of curry powder, with analysis by TD–GC–MS.
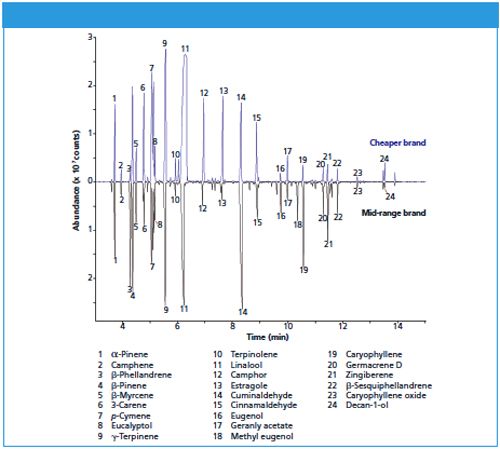
This analysis exemplifies how direct desorption can enable quick, robust analysis of multiple samples, which make it of considerable value to food analysts for quality control and product comparisons.
Typical Analytical Conditions
Sample: Curry powder (~50 mg), placed in an empty TD tube.
TD (TD-100): Tube: Desorbed at 50 °C (3 min). Trap (Material emissions): Analytes trapped at 10 °C, desorbed at 280 °C (5 min). Split ratio: Outlet 25:1.
Analysis: Single-quadrupole GC-MS operated in full-scan mode (m/z 45-600).

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).











