Quantifying Smoke Taint in California Wines by Immersive Sorbent Sheet Extraction Prior to Direct Analysis in Real-Time Mass Spectrometry (DART-MS)
Measuring volatile phenols in wine is essential in ensuring superior wine quality. A new analytical technique, called solid-phase mesh-enhanced sorption from headspace (SPMESH), was modified with direct immersion (DI) conditions and coupled to direct analysis in real time–mass spectrometry (DART–MS) to be used to detect smoke taint in winemaking.
The wildfires that hit northern California in 2020 caused extensive damage to numerous vineyards; however, in many cases, the vines themselves remained intact. Exposed to smoke for prolonged periods throughout the month of September, the 2020 harvest was written off by winemakers who were unwilling to risk producing wine with a smoky, ashy taint (1). This concern resulted in a major economic hit for an industry struggling to rebuild. Therefore, rapid and reliable methods for detecting compounds associated with smoke taint in unfermented grape juice or newly fermented wines are needed to help winemakers navigate this problem.
Grapes absorb volatile phenols, such as guaiacol (G) and 4-methylguaiacol (4-MG), from burning wood, storing them as nonvolatile glycosylated precursors prior to release during winemaking. Gas chromatography–mass spectrometry (GC–MS) techniques, utilizing solid-phase microextraction (SPME) fibers for phenol extraction, offer sensitive detection for such phenols but run times are in the order of 30–60 min per sample (2,3). This observation is an important limitation—for this application and more generally in the production of seasonal products—when analyses are required quickly and in large numbers, to support decisions around how best to manage the harvest at key points in the year. In 2020, winemakers needed reliable data to decide whether to discard fruit ahead of beginning the winemaking process but analytical demand rapidly outstripped supply as thousands of wineries sought smoke taint analysis via commercial laboratories with limited throughput. Inherently faster analytical techniques are needed to deliver higher throughput and ease this problem.
Direct analysis in real-time mass spectrometry (DART-MS) has a proven track record in the detection of food and drink contaminants (4) and the speed to deliver significant throughput gains to address analytical bottlenecks. However, off-aromas can result from odorants present at levels of just a few parts per billion or even less (5). Effective extraction and preconcentration of the odorants from the juice is therefore critical to capitalize on the inherent capabilities of DART-MS to provide rapid, sensitive detection.
Thin-film microextraction (TFME) coupled to ambient ionization (AI)-MS has already been successfully used to rapidly preconcentrate and quantify trace analytes present in complex matrices (2). TFME devices can be fabricated from adsorbent materials coated on solid substrates or absorbent polydimethylsiloxane (PDMS) mesh sheets—solid phase mesh enhanced sorption from headspace (SPMESH) sheets—with the latter exhibiting better performance for the concentration of volatile headspace compounds (6). Simple to prepare by laser etching, SPMESH sheets enable the simultaneous extraction of volatiles from multiple samples and have been successfully coupled with DART-MS to quantify µg/L to ng/L levels of odorant volatiles such as 3-isobutylmethoxypyrazine (IBMP, “green pepper”), linalool (“floral”), and methyl anthranilate (“Concord purple grape juice”), with speed and efficiency (7).
A direct immersion (DI)-SPMESH technique for the volatile phenols associated with smoke taint was developed and combined with DART-MS to provide a rapid, effective screening solution for assessing grape juice. A pragmatic alternative to the use of multipolymer SPMESH sheets, which are not commercially available, this technique removes any requirement for headspace partitioning and builds on previously reported success using DI for volatile phenols (8,9). Experiments were carried out to determine the selectivity of the resulting DI-SPMESH-DART-MS technique for the target phenols. The results show that this innovative approach offers advantages in regard to both speed and sensitivity compared with a headspace DART-MS technique and could be used to support winemaking decisions.
Developing a TFME Device for High Throughput Phenol Extraction
SPMESH sheets were made from PDMS sheets (Grace Bio-Labs) using laser etching to apply a 0.5 by 0.5 mm mesh pattern (Cornell NanoScale Facility) in accordance with a previously developed method (7). These were baked for 30 min at 250 °C prior to use.
For headspace (HS) extraction, samples were loaded into a 24 deep well plate (VWR), which was then covered with: a Teflon gasket; stainless-steel spacer; SPMESH sheet; second spacer; and stainless-steel top cover. Once secured, the assembly was shaken at 200 rpm at 50 °C for 30 min (C24KC Refrigerated Incubator Shaker).
For DI extraction, the previously described assembly was modified to include a second Teflon gasket between the spacer and top cover and secured using a standard woodworking vice clamp (Grainger). This complete assembly was then inverted and agitated at 200 rpm at 22.5 °C. Post-extraction the SPMESH sheets were rinsed with water to remove interfering analytes prior to DART-MS analysis (see Figure 1).
Figure 1: (a–e) A schematic of the developed DI-SPMESH-DART-MS method.
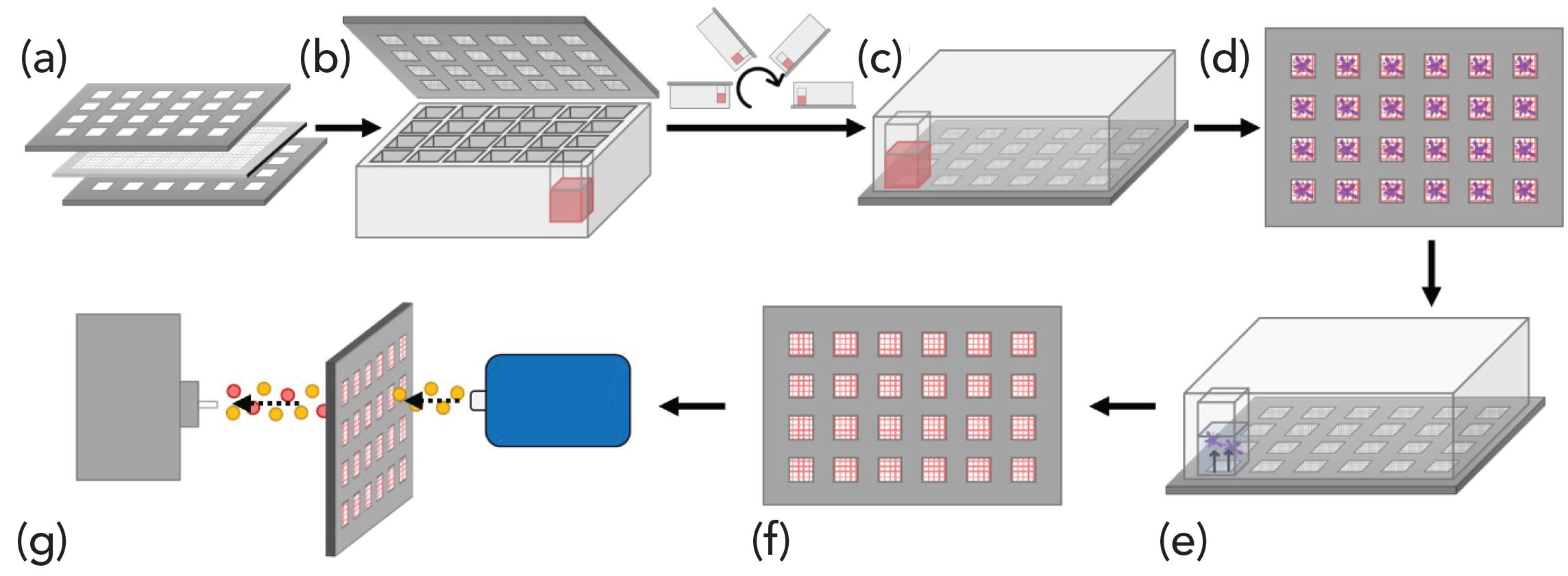
With both extraction methods SPMESH sheets are soaked in a water:methanol solution to remove residual non-volatiles prior to re-use.
Establishing a DART-MS Method for Fast and Reliable Analysis
In DART-MS, the analyte is volatilized and ionized at the inlet to a mass spectrometer by a heated gas flow, allowing analysis of a wide range of sample types without preparation under ambient conditions in an open environment. Here, the SPMESH sheets were placed on an automated positioning stage and scanned at a rate of 0.5 mm/s. Figure 2 shows a schematic of the standardized voltage and pressure (SVP) ion source used (DART, Ionsense Inc.). The high voltage needle applies an electrical discharge converting the inert gas, in this case helium, into a plasma. Ions and electrons are then filtered out, leaving only longer-lived excited atoms and molecules in the exiting stream. The grid electrode prevents ion-electron recombination and provides electrons for the negative-ion formation while the heater increases the temperature of the exiting gas, thereby promoting the desorption of molecules from the sample surface. The net result is a heated gas stream containing neutral, highly energetic atoms and molecules that interact with the sample to produce ions for analysis in a coupled atmospheric pressure ionization mass spectrometer.
Figure 2: A schematic showing the key features of an ion source for DART-MS.
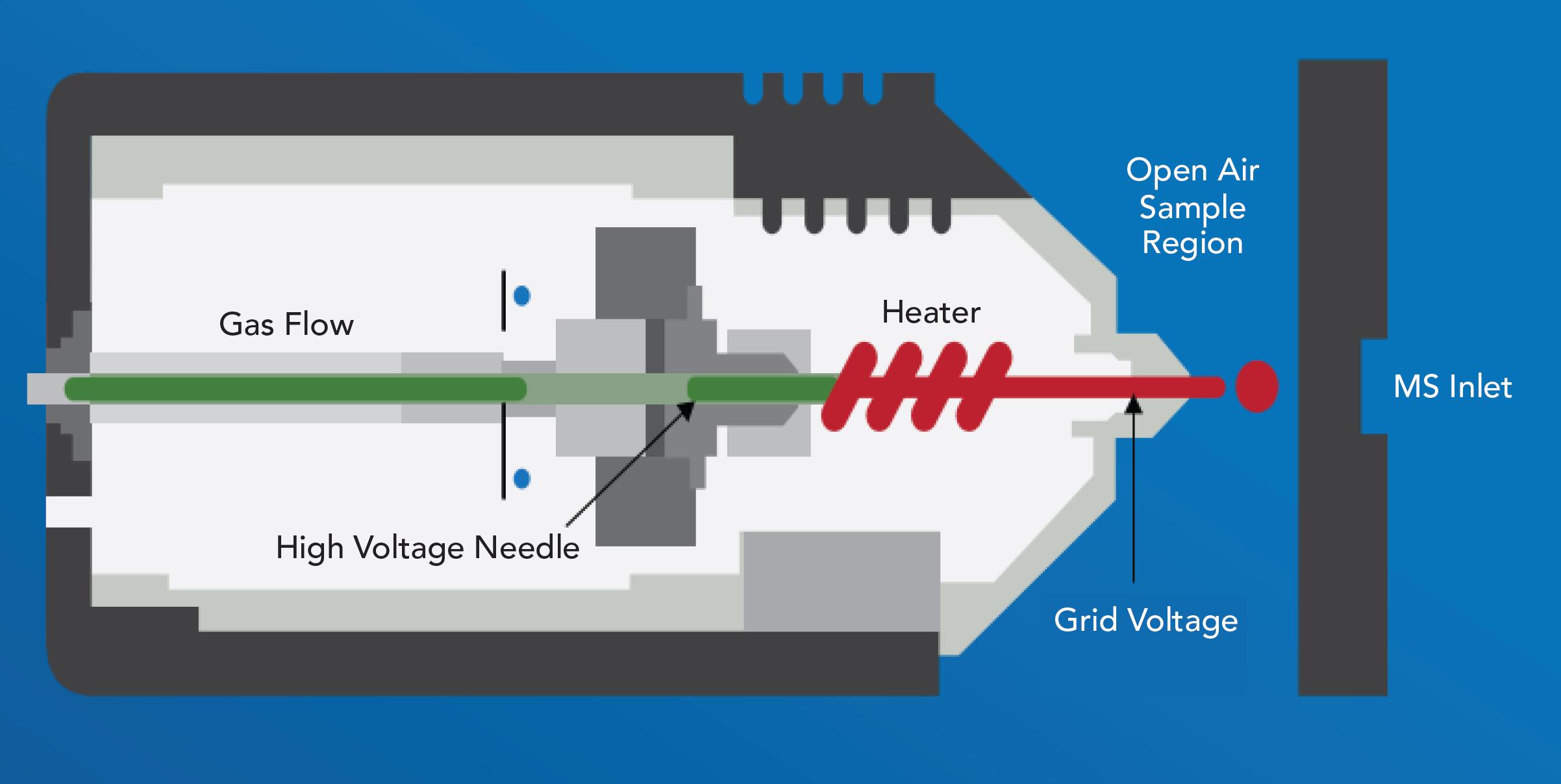
In this study the ion source was coupled to a triple quadrupole mass spectrometer (Finnigan TSQ Discovery Max, Thermo) via an interface (Vapur, IonSense) to maximize ion collection. Tests were carried out using two different ceramic caps—0.5 mm and 2.5 mm—at the outlet of the ion source to control the diameter of the gas stream, and both the ion source and mass spectrometer were operated in negative ion mode. The temperature of the exiting gas was 300 °C.
A range of baseline experiments and scans were carried out to identify precursors and products for each compound of interest and to establish a method for analyte quantitation. These included full scans of DI-SPMESH extractions of 10 mg/L aqueous solutions for each of the individual phenols of interest: guaiacol (G) (>99% purity), 4-methylguaiacol (4-MG) (99% purity) (Acros Organics), 4-ethylphenol (4-EP) (>99% purity, FCC, FG), and 4-ethylguaiacol (4-EG) (>99% purity, FCC, FG) (MilliporeSigma). To assess interferences between the compounds, DI-SPMESH extractions were also performed for 1 mg/L aqueous solutions of each the phenols of interest, for two deuterated internal standards—d4-4-ethylphenol (d4-EP) (> 98% purity, 99% D, Neta Scientific) and d3-guaiacol (d3-G) (>99% D, C/D/N Isotopes, Pointe-Claire)—and for syringol, a component of wood smoke. All measurements were made in triplicate.
Method Optimization
A series of tests were carried out to assess the integrity of the TFME device and to progressively optimize the method:
• To determine how watertight each of the two devices were, 5 mL of water was added to every other well (12 out of 24 wells in total). The device was then sealed, inverted, and visually checked for signs of leakage. Crosstalk, which is the extent of leakage of volatiles between the headspace of adjacent wells, was assessed by adding 5 mL of 10 µg/L IBMP in water solution to every other well and carrying out the HS-SPMESH-DART-MS technique as previously described (7).
• The stability of the analytes on the SPMESH sheets were assessed by carrying out HS-SPMESH extractions and leaving the sheets in air at room temperature for varying lengths of time—0, 20, 40 or 60 min—prior to DART-MS analysis.
• Desorption efficiency was assessed by carrying out HS-SPMESH extractions and then analyzing the same sheet by DART-MS three times in succession with no repeat extraction.
• Extraction kinetics were assessed by varying the incubation times used in both HS-SPMESH and DI-SPMESH extractions (six wells). Data were collected at incubation times of 5, 15, 30, 60, and 120 min.
• Sheet rinsing and sample dilution protocols were optimized by assessing the impact of rinse volume by immersing the SPMESH in 0, 1, 2, or 5 mL of water for 30 s following DI extraction of an undiluted spiked sample (standard solution of 4-EP, 4-EG, 4-MG and G (1 mg/L) in white grape juice (Welch’s)). Rinse time was also tested using a 5 mL water volume and a shaking time of 0, 10, 20, and 30 s and repeated with a diluted, spiked samples (1:1 with water).
Making Measurements
The developed DI-SPMESH-DART-MS technique was used to measure a range of commercial juice samples including white grape juice (Welch’s) and samples from local vineyards: Cabernet franc (Vitis vinifera), Chardonnay (V. vinifera), Concord (V. labruscana), Lemberger (V. vinifera), and Pinot Noir (V. vinifera). Comparative analyses were also carried out using the HS-SPMESH-DART-MS technique.
Limits of detection (LODs) were determined for the DI-SPMESH-DART-MS techniques in water and model juice. Well plates were pretreated with a 1 g/L bovine serum albumin solution to reduce active site binding for all measurements. Accuracy measurements were carried out using real juice samples spiked with target phenols.
Assessing Performance: Demonstrating an Effective High Throughput Technique for Volatile Phenol Detection
The experiments carried out enabled the progressive optimization of the DI-SPMESH-DART-MS method, ultimately resulting in a method with the sensitivity, accuracy, and throughput required to provide value for the application.
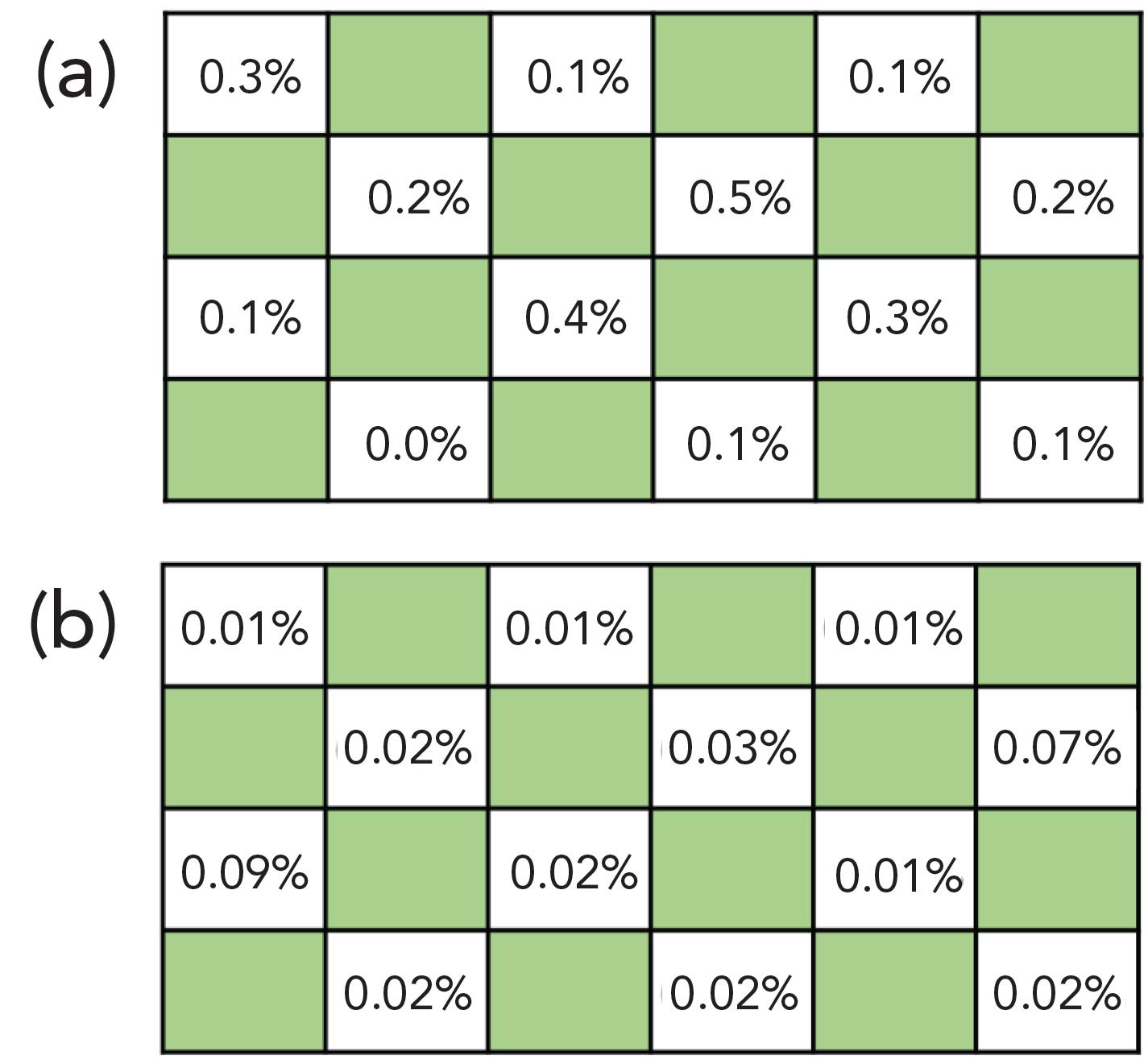
The design of the DI-SPMESH device was shown to be superior to that of the preceding HS-SPMESH assembly with respect to leakage and crosstalk. In leakage testing, the DI assembly remained watertight for 2 hr when inverted, whereas the HS design leaked, demonstrating its unsuitability for the DI technique. Crosstalk was an order of magnitude lower in the DI-SPMESH design (Figure 3b) relative to the original HS-SPMESH design which has levels ranging from 0.1 to 0.5% (Figure 3a). These results confirm the suitability of the new TFME device design for full utilization of the well plate and for the parallel extraction of volatiles onto spatially resolved locations on the sheet.
Figure 3: (a) The original HS design and (b) evidence of lower crosstalk with the new DI device indicates that all wells can be simultaneously used to maximize throughput in the parallel extraction of volatiles.
Each of the phenols was observed to form a series of oxygenated adducts of the form [M+nO-H]- (for example, [4-EP+O-H]-). These have potential to give rise to isobaric interferences among the analytes and steps were taken to refine the method for maximum selectivity with optimal m/z values selected for each of the phenols. Analytes were found to be highly stable on the SPMESH (no loss of volatile phenol signal after one hour in air), and the ion source was shown to be highly effective in achieving thermal desorption of the sample. An 80% reduction in signal was observed with each repeat measurement test in desorption studies. Taken together, these results demonstrate the potential utility of the DART-MS technique for extracted phenols.
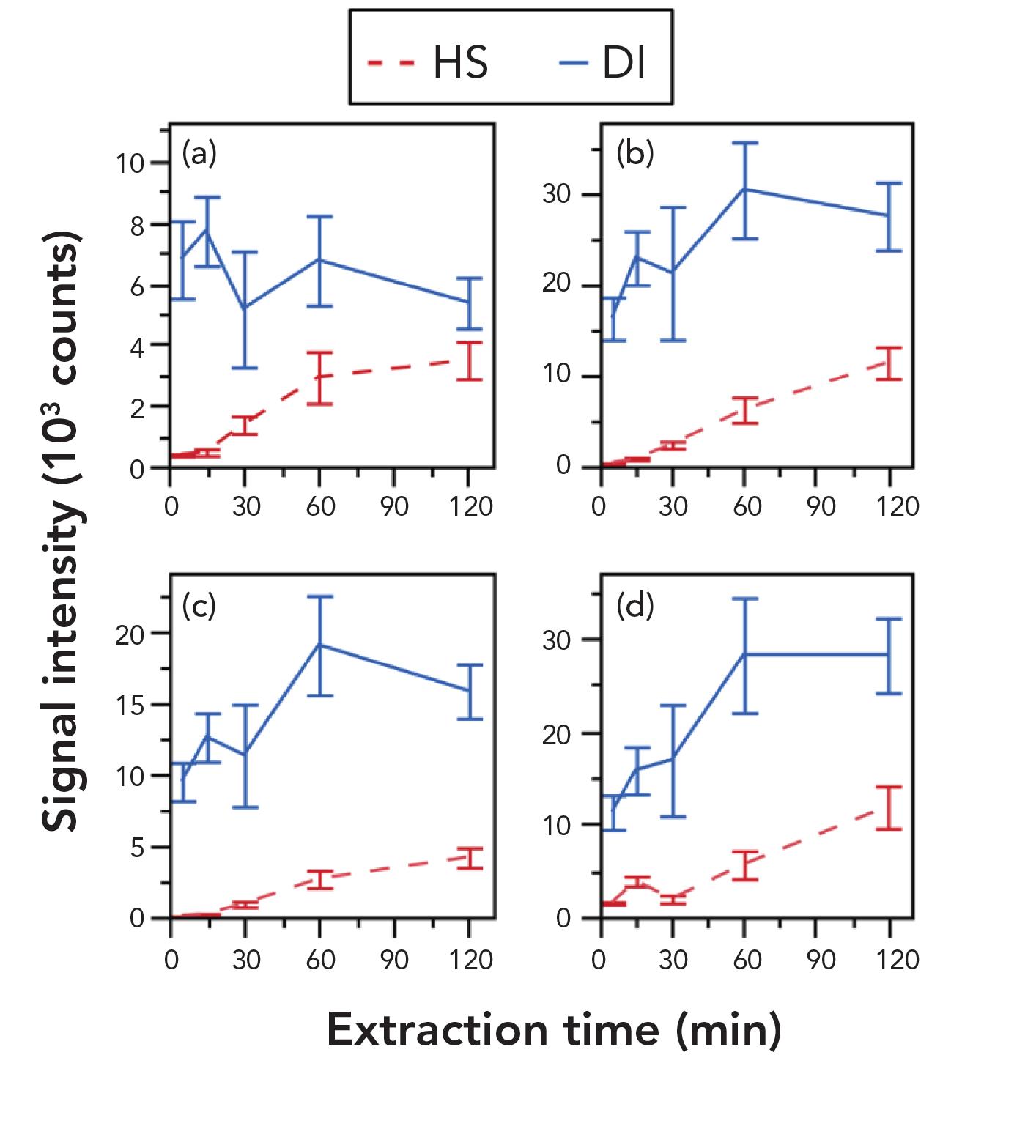
Initial studies with the HS-SPMESH device showed poor LODs for the target phenols in water and grape juice. Figure 4 shows the impact of longer extraction times and indicates that even after 2 hr, there is little, if any, evidence of a plateau in HS extraction (red line). DI extraction, in contrast, was found to be far more effective (see Figure 4—blue line). After 5 min, signal levels with DI match those achieved with HS after 120 min and equilibrium levels are reached within 60 min at most; the equilibrium signal intensities were far higher than those achieved with HS. These results provide further evidence of the superiority of DI for a high throughput technique.
Figure 4: The extraction of phenols—(a) G, (b) 4-MG, (c) 4-EP, and (d) 4-EG—is far more efficient with DI (blue) relative to HS (red), allowing extraction times to be cut to maximize analytical throughput.
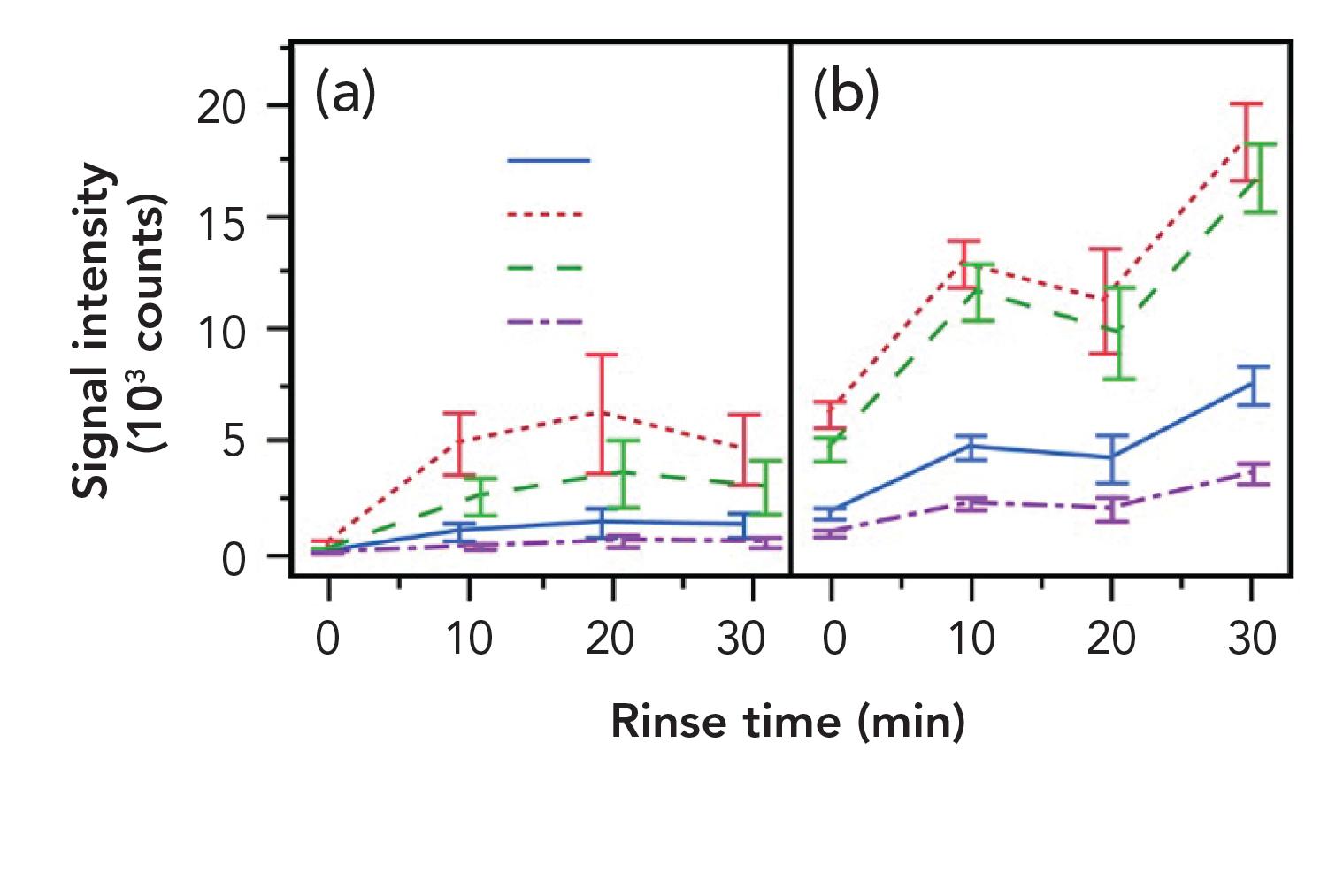
One final step with respect to optimizing the technique was to address the potential for fouling and associated interference from nonvolatiles associated with the repeated use of SPMESH sheets. No evidence of fouling was observed in the DI-SPMESH experiments. However, in initial experiments with phenol-spiked real juice samples, the volatile phenols proved undetectable, which was a result attributed to the adsorption of sugars, acids, and other juice components onto the surface of the SPMESH and the consequent competitive inhibition of volatile phenol ionization. Figure 5 shows how rinsing removes these polar compounds, thereby improving the signal. Following rinsing for 30 s in 5 mL of water, peaks associated with acid and sugar interference are no longer observed, and signal intensity for all the volatile phenols increases. Diluting the juice sample with water has an even more dramatic effect (see Figure 5b). Using a saturated sodium chloride solution for dilution also increased the signal, which was an effect attributable to the salting out phenomenon associated with this type of TFME device.
Figure 5: (a) Rinsing or (b) diluting samples are both effective in increasing the signal associated with the phenols of interest by reducing the impact of sugars, acids, and other juice components.
Based on these results, the optimized method included a two-fold brine dilution pre-extraction and a 30 s, 5 mL water rinse post-extraction. Table I summarizes the figures of merit for the fully optimized DI-SPMESH-DART-MS method and shows that the LODs for the four phenols in water lie between 0.5 and 1.7 µg/L; in model juice, the corresponding range is 0.9 and 2.8 µg/L. These compare well to quoted thresholds for sensory detection of the phenols which are in the range of 9.5–440 µg/L (4).
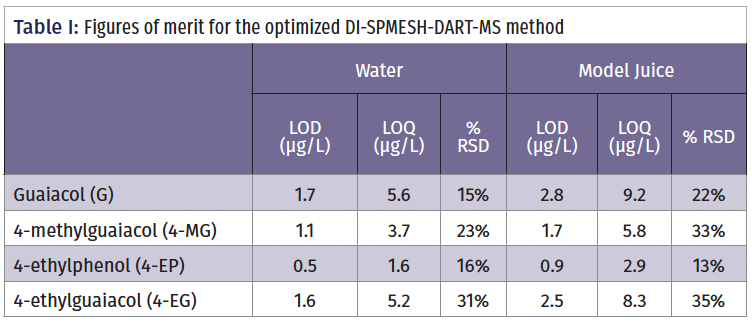
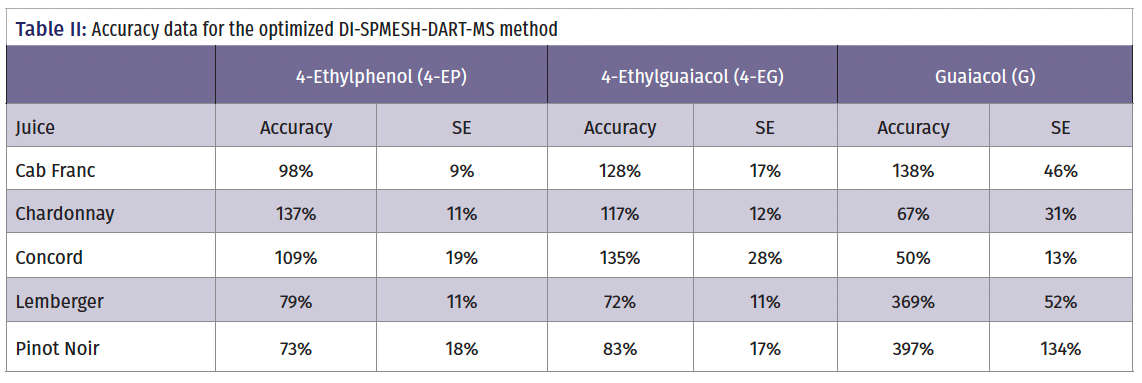
Table II summarizes the accuracy data for the method in different cultivars. 4-MG was not included in this analysis because of poor MS selectivity attributable to interferences from other compounds. With 4-EP and 4-EG, acceptable accuracy is observed with all grape cultivars (values in the range 72–137% of expected), but with G, accuracy is cultivar-dependent. Acceptable accuracy is observed with Cabernet franc and Chardonnay but not with Pinot Noir and Lemberger (>300%). G is also observed in unspiked samples of these cultivars, indicating a likely problem with isobaric interferences. Going forward, there is the potential to address this issue using alternative MS technologies (high-resolution MS), thereby expanding the range of cultivars for which the technique could be used.
In terms of throughput, the technique is significantly better than SPME-GC–MS alternatives with the capability to handle 24 samples in 45 min (that is, one sample per 2 min).
Conclusion
The method presented here provides a good example of the potential of DART-MS to provide practical and effective analysis for industrially relevant problems. A valuable technique for detecting volatile phenols associated with smoke taint in grape juice samples was developed by exploiting the inherent speed and flexibility of DART-MS. Using a DI-SPMESH extraction device, phenols can be simultaneously extracted with high spatial resolution to enable the full analysis of all 24 samples in just 45 min, which represents an order of magnitude improvement in throughput relative to established SPME-GC–MS techniques that typically take approximately 60 min for a single sample.
The limits of detection of the developed DI-SPMESH-DART-MS technique are in the low µg/L, below the sensory thresholds for the phenols of interest and good accuracy levels were achieved with multiple wine cultivars for the majority of the target phenols. These results suggest that the technique has considerable promise as a complement to GC–MS methods for rapid grape juice screening at periods of high demand. Specifically, commercial laboratories could use DART-MS as an initial high-throughput screening approach during periods of high demand, with GC–MS used for confirmatory analyses.
Acknowledgments
The figures and data were originally published in a paper in Journal of Agricultural and Food Chemistry.
References
- E. Asimov, “California Fires Take a Deep Toll on Wine Country,” The New York Times. Published Oct 5th 2020. https://www.nytimes.com/2020/10/05/dining/drinks/california-fires-wine-napa.html (accessed April 2022).
- N. Reyes-Garcés, E. Gionfriddo, G.A. Gómez-Ríos, M.N. Alam, E. Boyacı, B. Bojko, et al., Anal. Chem. 90(1), 302–360 (2018). DOI: 10.1021/acs.analchem.7b04502
- L. Maštovská and S.J. Lehotay, J. Chromatogr. A 1000, 153–180 (2003). DOI: 10.1016/s0021-9673(03)00448-5
- G. Tianyang, Y. Wei, Y.J. Yong, Z. Liya, L. Jiahui, W. Sai, et al, Mass Spec. Rev. 36, 161–187 (2017). https://doi.org/10.1002/mas.21466
- M. Parker, P. Osidacz, G.A. Baldock, Y. Hayasaka, C.A. Black, K.H. Pardon, et al, J. Agric. Food Chem. 60(10), 2629–2637 (2012). DOI: 10.1021/jf2040548
- J.P. Rafson and G.L. Sacks, J. Agric. Food Chem. 69(41), 12344–12353 (2021). DOI: 10.1021/acs.jafc.1c04197
- M.Y. Bee, J.A. Jastrzembski, and G.L. Sacks, Anal. Chem. 90(22), 13806–13813 (2018). https://doi.org/10.1021/acs.analchem.8b04465
- Q. Zhou, Y. Qian, M.C. Qian, J. Chromatogr. A 1390, 22–27 (2015). DOI: 10.1016/j.chroma.2015.02.064
- J. Marín, A. Zalacain, C. De Miguel, G.L. Alonso, and M.R. Salinas, J. Chromatogr. A 1098(1), 1–6 (2005). DOI:10.1016/j.chroma.2005.07.126
Gavin Sacks and Jessica P. Rafson are with Cornell University in Ithaca, New York. Jeffrey Zonderman is with IonSense in Saugus, Massachusetts. Direct correspondence to: zonderman@ionsense.com.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.









