Pursuing the Polar Routes with Penguins and Polar Bears: Balancing the Choice of Chromatographic Mode
Reversed-phase liquid chromatography (RPLC) may have historical popularity, but it is not the only LC technique available to chromatographers. To help balance the choice of chromatographic technique, a discussion of the merits of being flexible when making the choice of LC technique for a given application is presented. Because having a flexible perspective is particularly important for biotherapeutics, it is important that choosing which technique to use is determined based on good science and begins with a physicochemical property assessment of the analytes, followed by subsequent alignment with the most appropriate retentive mode. An Earth-based figurative analogy is used to help highlight several popular LC techniques.
Favoritism in daily decision-making has a profound influence in our lives as humans. The world of analytical chromatography is no exception, especially in the regulated space with the associated pressures for quality and timeliness. We want something that works as best as it can and arrives at a functional situation as soon as possible.
In my field of quantitative bioanalysis, which includes biopharmaceuticals to a continually increasing extent (in contrast to the world dominated by small-molecule therapeutic candidates of around 30 years ago), there is a limited range of options for liquid chromatography (LC) operation, and these options may be broadly classified simply as polar and nonpolar. In quantitative bioanalysis, the well-trodden path is in applying non-polar, better known as reversed-phase (RP) operation. To use an analogy—if you think of chromatographic operation as the Earth, RP operation may be seen as lying roughly at the equator. The conditions are nice, the land is well-mapped, there are lots of other people with similar interests around, and everything seems to be well-established and characterized. If problems arise with RP operation, there is confidence about how chromatographers can troubleshoot these issues.
Then, moving north or south, we enter the polar regions. These regions are far less explored and relatively sparsely populated. A good dose of mystery persists about how to negotiate our way through these parts of the world and, moreover, how to thrive if we decide we wish to get comfortable there. The poles of the Earth analogy as would befit the analysis of polar analytes correspond to polar modes of chromatography, dominated by hydrophilic-interaction chromatography (HILIC) (1). By contrast, as mentioned, the regions closer to the equator correspond to the RP domain, where we can relax and focus on purely dispersive interactions while sipping piña coladas.
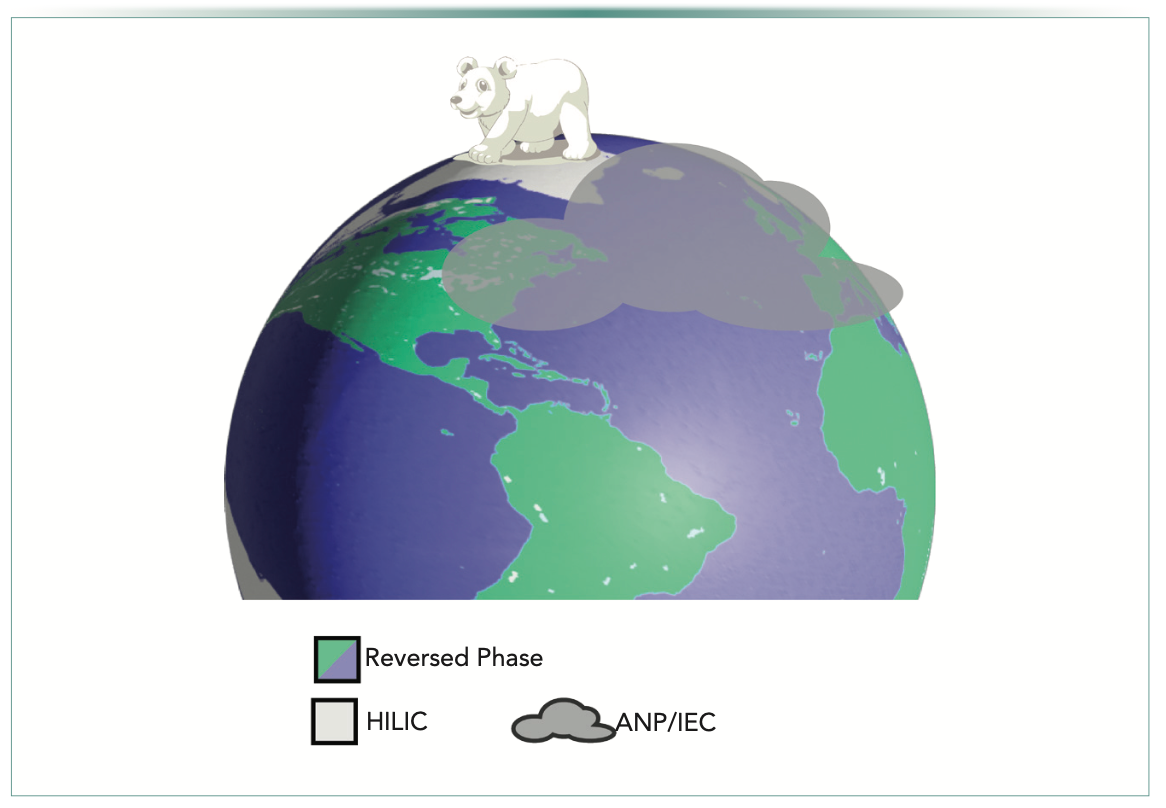
Moving between the poles and the equator, there are also fascinating areas corresponding to ion-exchange chromatography (IEC) and aqueous normal phase (ANP) chromatography, the bimodal function of silica-hydride–based column chemistries where the normal-phase modality can function simultaneously with the RP modality (2). The silica hydride surface can provide options in all chromatographic areas. Described in context with the Earth analogy, the snow-covered polar cap contrasts with the equatorial climes in terms of the diversity of available chemistries and the number of groups using that chromatographic mode. Figure 1 illustrates the analogy used in this article.
FIGURE 1: An analogy comparing the use of different modes of liquid chromatography (LC) to the geography of Earth. Reversed-phase (RP)-LC dominates in the more comfortable climes around the equator where nonpolar analytes are handled. Hydrophilic-interaction chromatography (HILIC) is most used in polar regions. Not discernible from either RP or HILIC and encompassing both are aqueous normal phase (ANP) and ion-exchange chromatography (IEC), which are used atop these regions (polar bear vector by Vecteezy.com; maps by FreeVectorMaps.com).
There is a great deal of comfort in RP operation. In chromatographic bioanalysis, users of LC–mass spectrometry (LC–MS) have roots in the small- molecule paradigm where they could so often use the RP modality with success. Hints of polar flavors could be added to stationary-phase chemistries, such as polar-embedded groups or polar endcapping, adjusting the selectivity and resolution as desired but without altering the fundamental mode of separation. To take the plunge into a different mode back in the days of small-molecule dominance, the options for unusual polar challenges were classical normal phase, which was sometimes used in tricky chiral applications, or HILIC. Between these, the only really practical option was HILIC, particularly given that it was the only option for which consumables and other support were readily available, and that it would not involve unfavorable operational aspects like system dedication because no dominant non-eluotropic solvent used in normal phase is water-miscible. Since approximately 2007, HILIC has modestly taken off in popularity in terms of the number of studies in the literature (3), and the increased usage of HILIC also corresponds to the increasing diversity of the stationary-phase chemistries available. As a result, the bioanalytical LC–MS community is becoming more familiar with the polar climes.
However, RPLC contains additional attributes that work in its favor in addition to the huge assets of familiarity, available phases, and knowledge base. RPLC is forgiving in various functional aspects, such as the tolerance—without chromatographic aberration—of a wide range of variability in the composition of injected solution. In analyzing bioanalytical extracts, decent sharp and symmetrical peaks, which are reflective of those from solution tests, may often be acquired in the crudest of extracts, and the columns maintain a good lifetime of more than one thousand injections. Additionally, the excursion in retention time with mobile-phase composition is significantly more linear and predictable than in normal-phase techniques.
All in all, reversed-phase LC is an old favorite and we can certainly understand the reasons why. Furthermore, the resistance to look toward other modalities strengthens in accordance with the affinity one feels towards reversed-phase. We have not only a wealth of different RP chemistries with advantageous polar incorporations available within the RP bracket, but we also have readily available ion-pairing reagents that can essentially make polar analytes nonpolar for the intended analytical endpoint of RPLC. However, ion-pairing reagents have several important disadvantages in LC–MS (4). The most notable of these disadvantages is its almost constant instrumental cleaning and concomitant downtime, short column lifetimes with permanent chemistry changes, and system dedication challenges. These considerations certainly impact the current biopharmaceutical analytical paradigm and will become more important in the future as LC–MS becomes more central as a bioanalytical platform, especially as instrument sensitivity continues to increase and as high-resolution MS technology becomes better aligned with the quantitative needs of chromatographers and is used more frequently.
Therefore, we can appreciate how RPLC is always attractive even though it is not always the ideal choice. Introducing ion pairing is a decision that, particularly in the world of LC–MS, should be carefully weighed against an alternative that may be suitable for the innate chemistry of the analyte molecular entities. In other words, a close look at physicochemical properties is certainly worthwhile.
A technique like HILIC may be appropriate more often than many may realize. In real projects involving new small-molecule drug candidates, we have frequently encountered that the presence of just one ionizable basic group is sufficient to enable HILIC retention, even if the remainder of the carbon skeleton bestows hydrophobicity. In antisense oligonucleotide work in the absence of ion pairing, our group found that HILIC worked emphatically well in quantitative bioanalytical LC–MS methods (5,6).
To put it simply, if an analyte or group of analytes is polar, there is a good chance that HILIC or another alternative to RPLC will work and provide the best results within the minimum time to complete. If the challenge involves hydrophobic analytes, then it is better to use RPLC as the first port of call in method development because that would be the best option. Because we know that “like dissolves like,” we can match polarity with chromato- graphic modality. With this logical basis, putting our proclivity for favorites aside, we can explore and become familiar with all areas of the chromatography world, even those that may seem frigid but are just waiting to be better characterized and have all their benefits revealed.
Acknowledgment
The author would like to thank Martin Squires for the illustration.
Disclaimer
The opinions expressed are solely my own and do not express the views or opinions of my employer.
References
(1) A. Alpert, J. Chromatogr. 499, 177–196 (1990). https://doi.org/10.1016/S0021-9673(00)96972-3
(2) J. Pesek and M. Matyska, Bioanalysis 4(7), 845–853 (2012). https://doi.org/10.4155/bio.12.39
(3) Y. Guo, Biomed. Chromatogr. 36(4), 1–25 (2021). https://doi.org/10.1002/bmc.5332
(4) M. Holcapek, K. Volnà, P. Jandera, L. Kolářová, K. Lemr, M. Exner, and A. Církva, J. Mass Spectrom. 39(1), 43–50 (2004). https://doi.org/10.1002/jms.551
(5) R. MacNeill, T. Hutchinson, V. Acharya, R. Stromeyer, and S. Ohorodnik, Bioanalysis 11(12), 1155–1167 (2019). https://doi.org/10.4155/bio-2019-0031
(6) P. Anand, M. Koleto, D. Kandula, L. Xiong, and R. MacNeill, Bioanalysis 14(1), 47–62 (2022). https://doi.org/10.4155/bio-2021-0216
Robert MacNeill is with the Department of Bioanalysis at Labcorp Drug Development Inc. in East Millstone, New Jersey. Direct correspondence to: robert.macneill@labcorp.com.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.










