PS-DVB Monolithic Columns Applied in A Microcolumn Switching Set-up for the Analysis of Peptides and Proteins
The Application Notebook
Polystyrene-divinylbenzene (PS-DVB) trap columns have been evaluated for microcolumn switching applications. In contrast to traditional stationary phases, which consist of packed particles, the monolithic separation medium is made of a continuous, rigid polymeric rod with a porous structure. The absence of intraparticular void volume increases separation efficiency, allowing for faster separations. Column lifetime is higher compared to packed columns.
Polystyrene-divinylbenzene (PS-DVB) trap columns have been evaluated for microcolumn switching applications. In contrast to traditional stationary phases, which consist of packed particles, the monolithic separation medium is made of a continuous, rigid polymeric rod with a porous structure. The absence of intraparticular void volume increases separation efficiency, allowing for faster separations. Column lifetime is higher compared to packed columns.
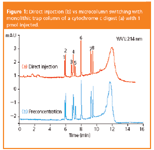
Figure 1: Direct injection (b) vs microcolumn switching with monolithic trap column of a cytochrome c digest (a) with 1 pmol injected.
This article demonstrates that the use of monolithic trap columns for preconcentration and desalting of peptides and proteins does not negatively influence chromatographic performance or sample recovery. Sample capacity of the monolithic trap columns (200µm i.d. × 5 mm) is 100 pmol for both peptides and proteins.
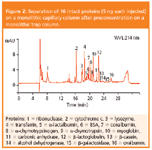
Figure 2: Separation of 16 intact proteins (5 ng each injected) on a monolithic capillary column after preconcentration on a monolithic trap column.
Experimental
LC system: UltiMate Plus nano LC system, Switchos
column switching module and FAMOS
autosampler (LC Packings/Dionex)
Trap column: Monolithic trap column, PS-DVB,
200-µm i.d. × 5 mm
Loading solvent: Water with 0.05% hepta fluorobutyric acid
(HFBA)
Loading solvent
flow-rate: 10µL/min
Analytical column: Monolithic capillary column,
PS-DVB, 200µm i.d. × 5 cm
Mobile phases: (a) Water, 0.05% TFA
(b) Water, acetonitrile (50:50%, v/v),
0.04% TFA
Flow-rate: 2.7µL/min
Gradient: 0–70% B in 7 min for peptides
30–100% B in 25 min for proteins
Column
temperature: 60 °C
UV detection: 214 nm; 3 nL flowcell
Direct Injection vs Microcolumn Switching
The influence of the monolithic trap column on chromatographic performance was evaluated by the separation of tryptic peptides of cytochrome c. Figure 1 shows a comparison between direct injection and microcolumn switching with a monolithic trap column. Table 1 lists peak widths at half height (PWHH) for the tryptic peptides with and without preconcentration.
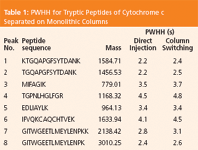
Table 1: PWHH for Tryptic Peptides of Cytochrome c Separated on Monolithic Columns
Protein Separation After Preconcentration Using Monolithic Columns
Figure 2 shows the separation of 16 standard proteins on a monolithic capillary column with sample loading on a monolithic trap column. PWHH were typically between 4 and 8 s.
Conclusion
The experiments demonstrate that monolithic trap columns can be used for preconcentration and desalting of samples consisting of peptides and proteins, without negatively influencing the chromatographic performance or recovery of the compounds. The sample capacity of the monolithic trap columns (200µm i.d. × 5Xmm) is in the range of 100 pmol for both peptides and proteins (data not shown).
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)







