Profiling of Biosimilars Using High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection (HPAE–PAD)
This article shows the potential of IC–ICP–MS for monitoring iodine-containing ionic oxidation by-products that form during ozonation of iodinated X-ray contrast media.
A large number of innovative biotherapeutics are losing patent protection, which fuels interest in the development of biosimilar therapeutics. A key step to proving biosimilarity is ensuring the correct N-glycosylations such as glycoprotein sialylations. This article reveals how high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE–PAD) can separate and identify carbohydrates with specific interactions between the hydroxyl and carboxyl groups of glycans based on charge, size, composition, isomers, and linkages without derivatization of samples. HPAE–PAD can separate glycans based on sialic acid linkage, which gives information about the carbohydrate sequence of a glycan, and also subtle linkage differences that may indicate disease states.
Photo Credit: GIPhotoStock/Getty Images

Patents for multibillion dollar biologics will expire over the next few years forcing biopharmaceutical companies to face an inevitable patent cliff because of a surge in the production of biosimilars.1 For a biosimilar to be approved, according to FDA regulations, it must have a highly similar structure and exhibit no clinically meaningful differences from the reference product in terms of safety, purity, and potency.2–3 Meeting these strict structural and clinical parameters can be very challenging because of the complex nature of biologics manufacturing.
The majority of biopharmaceuticals are glycoproteins manufactured via a complex process using recombinant DNA technology in live cells. During glycoprotein production proteins are glycosylated - an enzymatic post-translational modification process attaching glycans to proteins, which affects the final efficacy and safety of the product.4 Glycosylation affects biological activity, serum half-life, and immunogenicity, and depends on many manufacturing factors such as media, cell line, culture conditions, and bioreactors.4
Carbohydrates are difficult to analyze because they are highly polar compounds, exhibit similar structural characteristics among monosaccharides, and lack a suitable chromophore (unable to analyze with spectroscopy). High-performance anion-exchange chromatography (HPAE) takes advantage of the weakly acidic nature of carbohydrates to give highly selective separations at a high pH using a strong anion-exchange stationary phase.5 Coupled with pulsed amperometric detection (PAD), it directly quantifies non-derivatized carbohydrates at high femtomolar concentration levels with minimal sample preparation and cleanup.5
Most carbohydrates are not anionic at pH 7 and so must first become oxyanions in solution at a high pH (>12) to give an effective separation by anion-exchange chromatography. This is achieved by using hydroxide-based eluents accompanied with a stationary phase that can tolerate mobile phases of pH 12 and greater. After chromatographic separation, the non-derivatized carbohydrates are subsequently detected by PAD, a direct detection technique.
Alternative direct detection techniques available for liquid chromatography (LC) of carbohydrates are short wavelength ultraviolet light (UV) and refractive index (RI), but both lack the sensitivity required for analyzing mono- and oligosaccharides from glycoproteins.6 PAD applies a series of potentials (a waveform) applied two times per second (2 Hz) to a gold working electrode (WE) at high pH, resulting in the oxidation of analytes bound to the working electrode surface. PAD only detects compounds that contain oxidized functional groups at the detection voltage. Detection is sensitive and highly selective for electroactive species because many potentially interfering species cannot be oxidized or reduced, and are not detected.
Evaluating Protein Glycosylation Using HPAE–PAD
Comprehensive monosaccharide analysis helps to identify and quantify glycosylation of biosimilar proteins, thereby allowing researchers to plan more complex research in an informed manner. It is critical to analyze monosaccharides early on in process development to address any issues in the manufacturing process and prevent the manufacture of a defective product.
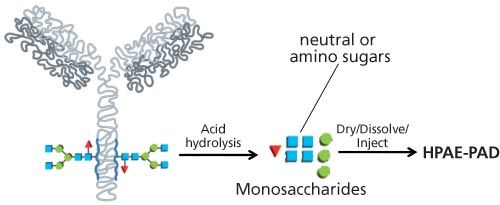
Sample Preparation: A sample of 0.67 μg protein serum album (PSA) and PSA treated with PNGase F were first hydrolyzed with slightly varied conditions (6N HCl) for neutral sugar or amino sugar analyses (Figure 1). The acid-hydrolyzed samples were dried in a SpeedVac concentrator (Thermo Scientific) equipped with an acid trap and reconstituted in a small volume of deionized water.6 Glycans released by PNGase F were identified by comparison to known standards and by enzymatic digestion to determine core glycan structures.
Figure 1: Schematic of simplified workflow for preparation of glycoprotein samples for HPAE–PAD monosaccharide separations and analysis.
Experimental Parameters:Columns: 3 × 150 mm, Dionex CarboPac PA20 (Thermo Fisher) and 3 × 30 mm, AminoTrap (Dionex); eluent: 10 min 100 mM KOH wash followed by 10 min of equilibration at 10 mM KOH; eluent source: Dionex EGC III and CR-ATC; flow rate: 0.5 mL/min; injection volume: 5 μL; temperature: 30 °C. Samples were hydrochloric acid hydrolysates of specified proteins. Peak results are shown in pmol.
Figure 2: Sensitive glycoprotein monosaccharide analysis using HPAE–PAD.
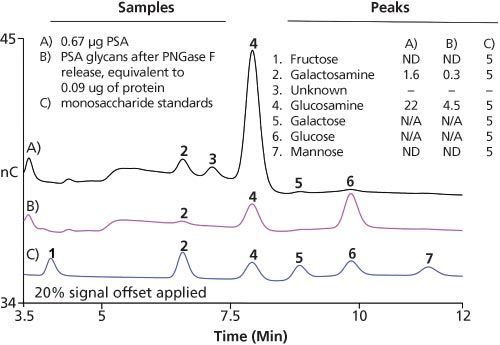
Results: Figure 2 shows the analytical sensitivity of monosaccharide analysis that can be achieved using HPAE–PAD. The monosaccharides present in both the treated and untreated PSA were compared with the monosaccharide standards, highlighting the sensitivity of HPAE–PAD. The loss of galactosamine and glucosamine after PSA treatment with PNGase were successfully identified. In both cases, the total protein hydrolysis and PSA-N-linked glycans had a similar ratio of 0.07 for gactosamine/glucosamine.
Sialic Acid Analysis
Unexpected sialylation of biosimilars can cause immunogenicity, so it is critical to monitor sialylation when changing process conditions. Determination of sialic acids in glycoproteins is not only performed to quantify the total sialic acid content, but also to determine relative amounts of N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc). Neu5Gc is undesirable in therapeutic proteins because of its potential immunogenicity and so must be tested for.6
Sample Preparation:The sialic acids, Neu5Ac and Neu5Gc, are negatively charged at pH 7 and acid-labile so require acetate in the eluent and weak acid conditions for hydrolysis. Before injection into the HPAE–PAD system, samples were subjected to acid hydrolysis or treated with neuraminidase to release the sialic acids, dried, and reconstituted in deionized water.
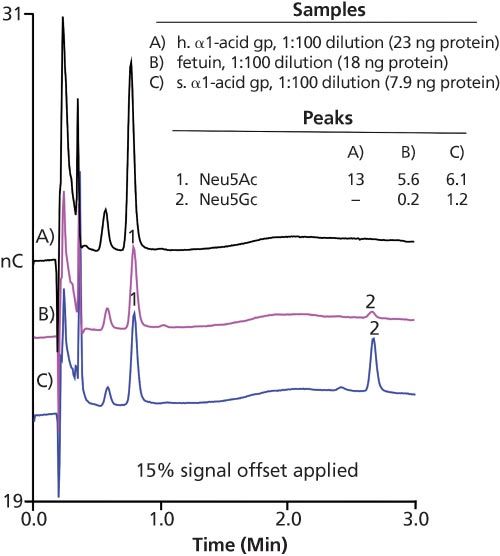
Experimental Parameters:Column: 3 × 30 mm, CarboPac PA20 Fast Sialic Acid (Thermo Fisher); eluent: 70–300 mM acetate in 100 mM NaOH from 0–2.5 min, 300 mM acetate in 100 mM NaOH from 2.5–2.9 min, 300–70 mM acetate from 2.9–3.0 min, 1.5 min of equilibration at 70 mM acetate in 100 mM NaOH; flow rate: 0.5 mL/min; injection volume: 4.5 μL; temperature:
30 °C; detection: PAD, Au on PTFE, 2 mil gasket. Peak results are shown in pmol.
Results: Figure 3 shows the quantitative determination of Neu5Ac and Neu5Gc using HPAE–PAD analysis for h. alpha-acid gp, fetuin, and s. alpha-acid gp. This information is critical in protein expression experiments for clonal selection in cell line development for therapeutic glycoproteins as well as for optimizing and monitoring protein production methods.
Figure 3: Fast separation of glycoprotein acid hydrolysates using HPAE–PAD.
Conclusion
In conclusion, a comprehensive characterization of the carbohydrate moieties in glycoproteins is an expected and necessary requirement to determine the development, efficacy, and safety of biosimilars. HPAE–PAD offers the most direct, sensitive, and selective method for monosaccharide, sialic acid, and other carbohydrate analyses required to understand glycosylation and related glycoprotein efficacy and immunogenicity.
References
- H.G. Grabowski, R. Guha, and M. Salgado, Health Affairs 33(6), 1048–1057 (2014).
- U.S. Affordable Care Act, Title VII, Sec. 7002(b)(3), pg 1823 (2010).
- S. Public Health Service Act, Sec. 351(i)(2) (2010).
- M. Angus and S. Elliott, Journal of Pharmaceutic Sciences94(8), 1626–1635 (2005).
- Verma, M., “HPAE-PAD for the Analysis of Carbohydrates” Thermo Scientific White Paper 70651 (2013).
- J.S. Rohrer, L. Basumallick, and D. Hurum, Biochemistry (Moscow) 78(7), 697–901(2013).
Parul Angrish is currently a product manager in the ion chromatography and sample preparation business unit at Thermo Fisher Scientific. She received her Ph.D. degree in organic chemistry from University of Florida. She has co-authored several peer-reviewed articles and is an inventor on several patents. Her current interest focuses on expanding the BioIC applications in pharma and biopharma markets using high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE–PAD).
E-mail: parul.angrish@thermofisher.com
Website: www.thermofisher.com

Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.
Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
This information is supplementary to the article “Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing”, which was published in the May 2025 issue of Current Trends in Mass Spectrometry.









