IC–ICP–MS for Monitoring the Fate of Iodinated X-ray Contrast Media after Ozonation
The Column
This article shows the potential of IC–ICP–MS for monitoring iodine-containing ionic oxidation by-products that form during ozonation of iodinated X-ray contrast media.
The combination of ion chromatography (IC) and inductively coupled plasma mass spectrometry (ICP–MS) provides a rapid, reliable, and sensitive speciation analysis. The method provides important information on the entry, degradation, and fate of various contaminants in (waste)water, as well as a reduction in sample preparation. This article shows the potential of IC–ICP–MS for monitoring iodine-containing ionic oxidation by-products that form during ozonation of iodinated X-ray contrast media, particularly, amidotrizoic acid and iomeprol. While amidotrizoic acid was completely mineralized, iomeprol degradation was not complete and proceeded via longer-lasting intermediates.
Contrast media is used in noninvasive diagnostics to assist targeted imaging (magnetic resonance imaging [MRI] and X-ray examinations) of organs and to differentiate between healthy and unhealthy tissue. MRI contrast media uses magnetic fields created by charged elementary particles, whereas X-ray contrast media, such as iodine and barium compounds, absorbs X-rays more so than body tissue creating a distinct contrast. Contrast media preparations are organically bound inert iodides that are almost all derived from triodobenzoic acid. They are safe to be administered intravenously or orally and are excreted unmetabolized from the body after a few hours. Because of their poor biodegradability, contrast media can enter the environment, even after passing through wastewater treatment plants, and can be detected at elevated concentrations. X-ray contrast media can be detected in most European receiving waters at μg/L levels.
Ozonation is an environmentally friendly chemical water treatment method that kills viruses, fungi, and bacteria, as well as degrading persistent organic pollutants (POPs). It does, however, have one drawback, which is that it can transform harmless substances into toxic compounds. In the case of iodized X-ray contrast media, it is not fully understood what happens during degradation and what oxidation products are produced. Ion chromatography–inductively coupled plasma mass spectrometry (IC–ICP–MS) is a technique that can be used to differentiate between various oxidation levels (speciation) and is therefore ideal for differentiating between free and bonded iodine ions. In this article, we performed IC–ICP–MS to determine iodized degradation products as well as inorganic oxidation products (iodate and iodide). Aqueous solutions of the model components iomeprol and amidotrizoic acid were subjected to different doses of ozone treatment to allow determination of residual X-ray contrast media and the degradation products.
Experiments
Ozonation:
Ozonation took place in an ozonation reactor (provided by the local authorities association for regional water supply) using aqueous solutions of 21.3 mg/L amidotrizoic acid (13.21 mg/L iodine) and 20.4 mg/L iomeprol (9.99 mg/L iodine), respectively. The ozone concentration in the ozone reactor was maintained at 3 mg/L and samples were taken at 30-min intervals over 3.5–4 h.
Separation Conditions:
The separation of the ionic, iodine-containing, degraded products took place under isocratic elution on a 250 mm × 4 mm Metrosep A Supp 3 column (Metrohm). The amidotrizoic acid and iomeprol were determined using a 125 × 4.6 mm Envirosep-PP RP-18 HPLC column (Phenomenex) under gradient elution. The quantification of both the X-ray contrast media and the degraded products was achieved using the standard addition method. The IC–ICP–MS analysis system consisted of an 850 Professional IC (Metrohm) and a VG PQ ExCell ICP–MS (Thermo Scientific). The determination parameters of the ICP–MS are shown in Table 1.
IC Parameters:
Eluent A: 0.1% formic acid in water (v/v), Eluent B: acetonitrile, 0.5 mL/min, gradient: 0–0.5 min: 95% A, 5% B, 0.5–5.7 min: 60% A, 40% B; 5.7–10 min: 95% A, 5% B. b) course of the peak area of the second OBP-peak (1:100 dilution) depending on the ozonation time.
Results
Amidotrizoic Acid:
Despite its ionic character, amidotrizoic acid could not be analyzed as an evaluable peak using IC–ICP–MS; however, iodate was detected as the only ozonation product.
Figure 1: Recovery rate of the iodine in the form of iodate and amidotrizoic acid depending on the duration of the ozonization process.
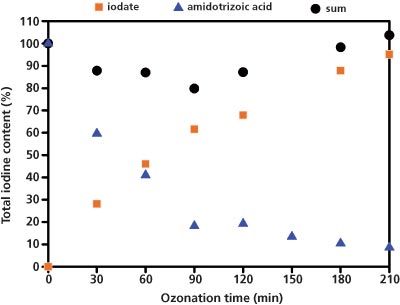
Figure 1 shows that after 210 min, 95% of the total amount of iodine present was in the form of iodate. Liquid chromatography coupled to UV (LC–UV) analysis carried out in parallel on the remaining amidotrizoic acid showed that after 210 min only 8% of the total amount of iodine was still present as amidotrizoic acid. The recovery rate of 103% of the iodine excludes any further ozonation products.
Iomeprol:
Iomeprol degraded considerably slower than amidotrizoic acid - only 14% of the iodine present was in the form of iodate after 210 min. LC–UV analysis carried out on the remaining iomeprol showed only 16% of the iodine was in the form of iomeprol, meaning that the total recovery rate was only 30% (Figure 2). The rest of the iodine must therefore be present as various degradation products.
Figure 2: Recovery rate of the iodine as iodate and iomeprol depending on the duration of the ozonization process.
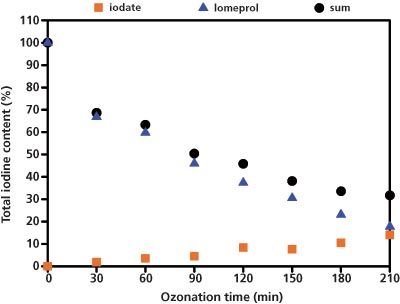
Figure 3: IC–ICP–MS chromatogram of a 100 μg/L iodate and iodide standard in comparison with an iomeprol solution after 60 and 210 min ozonation process, respectively.
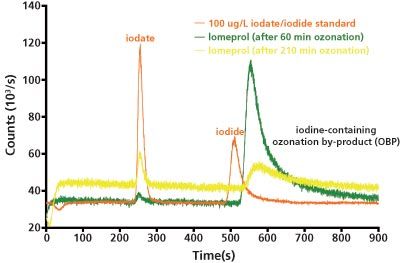
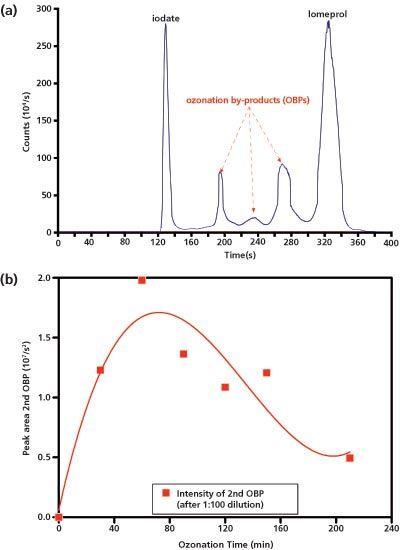
A comparison of chromatograms of a 100-μg/L iodate/iodide standard with the 60 min and 210 min ozone-treated iomeprol solution shows that the second peak could not be that of the earlier eluting iodide because of the significant differences in the retention times. The high performance (HP)LC–ICP–MS measurement presented in Figure 4 suggests that one or several ionic degradation products of the iomeprol cause this peak. An exact quantification of this peak was not carried out. On lengthening the duration of the ozonation, an increase in the peak area can be observed; this decreases again after 60 min. This seems to be evidence of a further iomeprol oxidation product that builds up quickly initially but, with further ozonation, is once again degraded.
Figure 4: (a) HPLC–ICP–MS chromatogram of an iomeprol solution after a 120 min ozonization. (b) Course of the peak area of the second OBP-peak (1:100 dilution) depending on the ozonization time.
Conclusion
IC–ICP–MS analysis showed that it is possible to determine the effect of ozone treatment on iodinated X-ray contrast media from the amount of iodate produced. While a 210-min ozonation treatment degrades almost all of the amidotrizoic acid to iodate, under the same ozonation conditions 16% of the iomeprol is still present. Only 14% is present as iodate, and in the ion chromatogram other, yet to be identified, peaks occur that are assumed to be other iodine-containing degradation products. That being said, under the chosen ion chromatographic conditions, it is not possible to document the intact iodinated X-ray contrast media.
Reference
- W. Seitz et al., Chemie in Labor und Biotechnik12, 456–460 (2004).
Peter Pfundstein
has worked as a laboratory engineer as a specialist for different kinds of analytical techniques at Aalen University since 2009. After his education as “Chemisch-technischer Assistent”, he studied chemistry at the “Hochschule für Technik und Wirtschaft Aalen” (Germany). He obtained his engineering degree under Prof. Dr. Dirk Flottmann in analytical chemistry.
Christian Martin
has worked since 2013 as a laboratory specialist at Institute Dr. Nuss, Bad Kissingen. Prior to this he worked at the Leibniz Center for Tropical Marine Ecology. His main focus is in the area of element analytics (ICP, IRMS). Previously he studied chemistry at the “Naturwissenschaftlich-Technische Akademie Isny“ (Germany) and at the “Hochschule für Technik und Wirtschaft Aalen” (Germany). He obtained his Masters under Prof. Dr. Dirk Flottmann in analytical chemistry using IC–ICP–MS-coupling techniques.
Wolfgang Schulz
studied chemistry at the University Aalen and physics at the University Stuttgart. He received his Ph.D. from the University Lüneburg. He is head of the laboratory at the Zweckverband Landeswasserversorgung in Langenau, Germany.
Wolfram Seitz
studied chemistry at the University of Applied Sciences Aalen, Germany, from 1999 to 2003. From 2004 to 2007, he pursued a programme of study in the field of water chemistry and was awarded the degree of Doctor in Philosophy by the University of Surrey, UK, in January 2008. Since 2004 he has been working as an analytical chemist at Landeswasserversorgung, Langenau, Germany.
Katinka Meike Ruth
is product specialist in the Competence Centre for Ion Chromatography at Metrohm. She studied chemistry at the Technical University of Munich, Germany, where she obtained her Master’s qualification with majors in organic chemistry and biotechnology. Katinka’s Ph.D. was conducted at the Swiss Federal Institute of Technology and the Swiss Federal Laboratories for Materials Science and Technology. Before joining Metrohm, she worked as scientific assistant at the Swiss Federal Institute of Aquatic Science and Technology.
Andrea Wille
is manager of the Competence Centre Ion Chromatography at Metrohm International Headquarters in Herisau, Switzerland. Previously, she worked as product manager for ion chromatography and hyphenated techniques at Metrohm AG. She studied chemistry at the TH Merseburg and the University of Halle-Wittenberg, Germany. She obtained her diploma and Ph.D. in analytical environmental chemistry. Before joining Metrohm AG, she worked as a scientific assistant at the Alfred-Wegner-Institut für Polar- und Meeresforschung Bremerhaven, Germany.
Alfred Steinbach
is technical writer in the Marketing Department of Metrohm AG, based in Herisau, Switzerland. He obtained his Masters in nuclear chemistry from the University of Cologne, Germany, and his Ph.D. in environmental and analytical biogeochemistry from the University of Hamburg, Germany. Before obtaining his Ph.D. and joining Metrohm AG in 2006, he carried out research in nuclear, analytical, and environmental chemistry and worked as production manager at BASF Venezolana S.A.
Dirk Flottmann
is professor for analytical chemistry at Aalen University since 2002. Previously he worked at Wacker Chemie (Burghausen, Germany) and Wacker Siltronic (Singapore) as laboratory manager in the semiconductor area. He studied chemistry at University of Bielefeld (Germany) and obtained his Diploma and Ph.D. degree in the field of physical analytical chemistry. Present research topics are in the field of ultratrace analytical method development by ICP–MS and in organic trace analytical work.
E-mail: ast@metrohm.com
Website: www.metrohm.com

Maximizing Cannabinoid Separation for Potency Testing with LC
April 7th 2025Researchers from the Department of Chemistry at Western Illinois University (Macomb, Illinois) conducted a study to optimize the separation of 18 cannabinoids for potency testing of hemp-based products, using liquid chromatography with a diode array detector (LC–DAD). As part of our monthlong series of articles pertaining to National Cannabis Awareness Month, LCGC International spoke to Liguo Song, the corresponding author of the paper stemming from this research, to discuss the study and its findings.
How Many Repetitions Do I Need? Caught Between Sound Statistics and Chromatographic Practice
April 7th 2025In chromatographic analysis, the number of repeated measurements is often limited due to time, cost, and sample availability constraints. It is therefore not uncommon for chromatographers to do a single measurement.










