Professor Barry L. Karger: Scientist, Mentor, and Innovator
LCGC North America
A review of Professor Barry L. Karger's years as a teacher and mentor, as well as his many scientific accomplishments
Professor Barry L. Karger, one of the earliest contributors of modern high performance liquid chromatography (HPLC) and bioanalytical separations and analysis, recently celebrated his 50th year as professor of chemistry at Northeastern University in Boston, Massachusetts, and his 40th year as Director of the Barnett Institute of Chemical and Biological Analysis. His years as a teacher and mentor to scores of students, many of whom hold key positions in industry and academia, as well as his accomplishments in the scientific arena, are reviewed by Howard Barth, his former student.
In 2013 Professor Barry L. Karger celebrated his 50th anniversary as professor of chemistry at Northeastern University (NU), and his 40th year as Director of the Barnett Institute of Chemical and Biological Analysis (Figure 1). Karger has influenced the lives of hundreds of PhD graduate students, postdoctoral fellows at NU, and staff scientists at the Barnett Institute. He was responsible for developing two generations of analytical chemists, as well as training many students in cutting-edge separation science and bioanalytical chemistry. His research programs have attracted many high-level and prominent scientists who have spent their sabbaticals in his laboratories, advancing analytical sciences. At 74 years of age, Karger is still actively writing, lecturing, and promoting the study of bioanalysis.

Figure 1: Professor Barry Karger surrounded by high performance liquid chromatography (HPLC) systems at the Barnett Institute.
It was also 50 years ago when our paths first crossed. I was a high school senior in Boston, Massachusetts, and Karger was a newly appointed assistant professor at NU. I had just placed first in the regional and state high school science fairs for analyzing trace metals in seawater (Boston Harbor), using a recently introduced chelating resin for removal and concentrating metal ions and a homemade polarograph for detection, which forever fueled my interest in analytical chemistry. My enthusiasm knew no bounds, and after a few phone calls, I reached Karger at NU and asked if he would take me on as a summer student in his laboratory. Although he was just setting up his programs, he invited me to visit when I enrolled at NU in 1964. That was when our first interaction began, which continued throughout most of my time as an undergraduate, and for many years after I earned my PhD from Karger. It was through him that I obtained my first job in industry as a chromatographer.
In recognition of Karger's scientific accomplishments, and the impact that he has had on the development of such a large number of analytical chemists, this biography is dedicated. (Because it is not practical to list all of Karger's patents and publications, only a select few are referenced to give the readers a flavor of his output.)
Early Years as an MIT Undergraduate
Karger's first exposure to separation science was during his undergraduate years as a chemistry major at Massachusetts Institute of Technology (MIT) in Cambridge, Massachusetts. At the time, MIT had an active analytical chemistry department, headed by Professor L.B. "Buck" Rogers, one of the "fathers" of analytical chemistry. For his senior research project, Karger was introduced to foam fractionation by Rogers. This method is an offshoot of ore flotation, a mining operation in which solutes of interest are selectively adsorbed onto surface-active bubbles forming solute-enriched foam that is continuously collected and processed. Nowadays, bubble adsorptive methods have been replaced with modern separation techniques.
Karger published his first paper in 1961 on the foam fractionation studies he had performed for his senior research project with Rogers at MIT (1). This initial exposure to separation science decided his fate. He was not only attracted to the experimental and theoretical aspects of the separation process, but also to physicochemical measurements that could be made with chromatographic techniques.
Graduate school was next. Most universities at the time had analytical programs in electroanalytical chemistry, spectroscopy, or wet-chemical methods. Rogers, however, strongly suggested that Karger focus on research in chromatography, particularly gas chromatography (GC), an emerging technology that offered many opportunities regarding theory and applications.
Gas Chromatography at Cornell University
There were only a few graduate programs in the early 1960s that offered studies of separation science. One such program was at Cornell University, headed by Professor Don Cooke, one of the more popular analytical chemists at the time. In 1960, Karger enrolled at Cornell, and within three years of dedicated research, he had produced a series of papers on the influence of GC column and operating parameters using time normalization. This method was a new approach for optimizing the separation process and has since been adapted by many other investigators. Karger also designed and built a new version of a flame ionization detector for GC.
After receiving his PhD in 1963, he and his wife Trudy decided to move back to Boston to be closer to family. Because of the baby boom, universities were expanding their departments and teaching positions were plentiful. He accepted a faculty position at NU, noted for its cooperative work and study program for undergraduates. It was an opportunity for Karger to establish a research program dedicated to separation science, a growing discipline that was demanding more trained chemists.
The Northeastern University Years
Within the first few years of his arrival at NU, Karger was awarded sufficient research grants to establish concurrent programs involving four areas of research: adsorptive bubble fractionation (his group was affectionately called "Barry and the Bubblers," after rock groups of the time); theoretical aspects of chromatographic separations; thermodynamic measurements using GC; and GC separation of stereoisomers.
Besides developing active research groups, he also established graduate-level courses in separation science and advanced topics in analytical chemistry. By the end of the 1960s, Karger had become very active in the theoretical and practical applications of analytical separations. His graduate programs attracted a great deal of interest, and his courses and lectures on separation science were always well attended — Karger has always been a dynamic and enthusiastic speaker.
Undergraduate Years with Karger
During my undergraduate years, Karger either assigned me small projects or allowed me to assist his graduate students. For example, I measured height equivalent to a theoretical plate (HETP) values manually for Karger's optimization studies, as laboratory computers had not been developed. Soon I became a "bubbler," setting up foam fractionation experiments. Even though I had not taken a formal organic chemistry course, Karger trusted me to synthesize a new chelating surfactant for foam fractionation, but as much as I tried, I was never able to recover crystals from the oily product that was formed.
In my junior and senior undergraduate years, I was pushing back the "frontiers of science" by applying GC to the study of structural changes of liquid crystals when used as the stationary phase of packed columns. Although we were able to monitor morphological changes of liquid crystalline compounds from retention factor measurements, reproducibility was a problem because of supercooling of some compounds. (The valuable lesson learned was to never repeat an experiment if the initial data look good!)
In 1969 when it was time to choose a graduate school, I was happy to stay on at NU and begin research into high performance liquid chromatography (HPLC) with Karger as my mentor and research advisor.
HPLC at NU
Early studies involving column chromatography had shown that small-particle-size packings resulted in orders of magnitude improvement of resolution, as predicted by the van Deemter equation. In the early to mid-1970s, 35-µm silica particles were the standard particles, followed by 10-µm and 15-µm particles. With HPLC columns, total analysis time was reduced from hours to minutes. It was obvious to Karger, with encouragement from Csaba Horváth, of Yale University in New Haven, Connecticut, that the future of separation science lay in the development of high performance LC packings and improved instrumentation, automation, and data processing. Clearly, a better understanding of the separation mechanisms and peak broadening was required.
In the late 1960s, Karger phased out of adsorptive bubble fractionation and geared up for HPLC. As he once reminisced, "I often think about my early days involving foam fractionation when I have a beer." Since his GC studies were well-funded, and generating a great deal of useful information, he kept those programs active until the mid-1970s.
Graduate Studies with Karger
As a graduate student from 1969 to 1973, I focused on fundamental HPLC studies using liquid–liquid chromatography, since bonded phases were yet to come. My research involved an investigation of factors affecting HPLC column resolution and peak broadening (2). A separate study was conducted on the reproducibility and accuracy of HPLC analysis using an on-line dedicated computer with software developed at NU, a major innovation at the time (3).
During this period, we coated and packed our own HPLC columns. Because of the expense of silica, the recovered packing was reused after decomposing the stationary phase with sulfuric acid, followed by exhaustively washing with water. For pumping systems, we used either a long coil of large-internal-diameter stainless steel tubing filled with mobile phase and pressurized with a tank of nitrogen, or one of several available modified industrial pumps. In the early 1970s, James Waters, founder of Waters Associates (now Waters Corporation), provided Karger with one of the first constant-flow-rate HPLC pumping systems, which was shared among graduate students.
The close interaction and friendship begun at that time between Karger and Jim Waters led to an endowed chair that Karger has held since in 1985 (see Figure 2). Karger is most proud of this honor, especially when he is introduced at scientific meetings as the James L. Waters Chair in Analytical Chemistry.

Figure 2: Professor Barry L. Karger (left) receiving the James L. Waters Chair of Analytical Chemistry in 1985 from the late NU president Kenneth Ryder (center) and James L. Waters (right), founder of Waters Associates.
Barth Leaves Boston for Philadelphia
In 1973, after leaving graduate school, I was awarded a postdoctoral fellowship at the Institute of Clinical Sciences at Hahnemann Medical College in Philadelphia, Pennsylvania. After my stay at Hahnemann, Karger was kind enough to help me obtain a position as an industrial analytical chemist at Hercules Inc., in Wilmington, Delaware, where I practiced my craft as a chromatographer for the next 14 years. Then in 1988, I joined the Central Research Department of DuPont Company, and retired in 2007 as a senior research associate.
At the Hercules Research Center (now Ashland Chemical Inc.), I became involved with size-exclusion chromatography (SEC) and polymer characterization and was appointed group leader of separation science and particle size measurements. These interests continued at DuPont Experimental Center where I initially worked on projects involving SEC-online viscometry and light scattering with Wallace Yau. Somewhat later, I was assigned to special projects involving SEC, HPLC, temperature rising elution fractionation (TREF), and trace analysis in support of many of DuPont's business centers. I continued to be active in professional societies, most notably the American Chemical Society (ACS) and the International Symposium on Polymer Analysis and Characterization.
On the Fast Track
Because of Karger's popularity and reputation as a prodigious writer and productive scientist at the cutting-edge of analytical chemistry, he had no difficulties attracting graduate students and visiting scientists, as well as funding. Throughout the years, he brought in distinguished visiting scientists and postdoctoral fellows to augment his programs and provide his graduate students with added technical guidance and resources.
Advancements came fast. In 1970, Karger was promoted to associate professor, and in 1973 to full professor of chemistry. In that same year, he co-authored, with Lloyd Snyder and Csaba Horváth, the popular book An Introduction to Separation Science (4). For the next 25 years, it was the standard graduate text for separation science, covering all modes of analytical separations.
Because of Karger's hectic schedule, his well-earned sabbatical was postponed until 1973, at which time he worked with Georges Guiochon at the Ecole Polytechnique in Paris, France, followed by several months with Istvan Halasz and Heinz Engelhardt at the University of Saarbrucken in Germany. Guiochon and Halasz reciprocated by teaching graduate courses at NU, and Engelhardt was Karger's first postdoctoral fellow in 1969. Engelhardt, by the way, was trained as an organic chemist and proved to be a popular and helpful resource for graduate students. Karger's second (and last) sabbatical was taken in 1979, during which time he taught and worked with Emanuel Gil-Av at the Weizmann Institute of Technology in Israel, on the use of HPLC for chiral separations.
HPLC Mechanisms and Applications
Karger continued his studies of GC adsorption and partition mechanisms of separation, but by the mid-1970s he focused his attention on HPLC separation mechanisms (5–8) and clinical chemistry and biochemical applications. For example, ion-pair partition chromatography was optimized and applied to the separation of biogenic amines, sulfa drugs, and thyroid hormones. Different precolumn derivatization methods were evaluated and applied to amino acid analysis and optically active amino acid derivatives (9). Figure 3 shows an early example of the separation of optically active amino acids in which the mobile phase included an optically active additive (chiral chelate) (9).
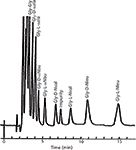
Figure 3: HPLC separation of 5-dimethylaminonaphthylene-1-sulfonyl (Dns) glycyl-D,L-amino acid dipeptides with a mobile phase consisting of acetonitrileâaqueous ammonium acetate containing L-2-ethyl-4-octyl-diethyltriamine-Ni(II) chelate and a C8 reversed-phase column. Adapted and reproduced with permission from reference 9, copyright Elsevier Publishing.
In the 1980s, Karger became interested in liquid chromatography coupled to mass spectrometry (LC–MS) for determining low levels of biological compounds and metabolites, as well as for identifying the chemical structure of peptides. In view of his leanings, Karger teamed up with his NU colleague, Paul Vouros, a noted mass spectrometrist, and together they published many papers in this area. During this period, Karger also demonstrated the kinetic denaturation of proteins on hydrophobic surfaces (10). His group also showed that SEC columns packed with ether-bonded silica could be used at high antichaotropic salt concentrations to enhance peptide and protein hydrophobic retention (11). These studies helped to pave the way for hydrophobic interaction chromatography (HIC), which is widely used for the purification of biopharmaceuticals as the proteins can be recovered in an active state.
New Challenges: Bioanalytical Separations
The entire makeup and function of all living systems are governed by the chemical composition and concentration of potentially thousands of different proteins, all of which are genetically expressed. In addition, there are numerous post-translational modifications. For many decades, it has been known that chemical composition and concentration of proteins determine the health, function, pathology, and longevity of the organism. To correlate cellular functions and physiology with genetic information, we must be able to separate and analyze exceedingly low concentrations and amounts of proteins found within cellular material. This information is required to determine the onset of disease states and to develop drugs to alter biochemical pathways.
The daunting task facing chemists was to develop the tools necessary to sift through biological material to determine the composition and levels of proteins. In the 1980s, Karger took on these challenges, focusing his research on high-resolution, multidimensional separations with on-line mass spectrometry.
In this regard, I must note that one of Karger's admired traits is his flexibility to keep current and his willingness to risk entering into new fields of study, an attribute he has shown throughout his professional career. Within a short time frame, Karger was able to acquaint himself with current bioanalytical literature, identify key analytical problems, and map out strategies for complex bioseparations, including detection modes. To develop a team of scientists with the appropriate backgrounds, he sought out graduate students interested in bioanalytical chemistry, attracted well-known visiting life scientists, and developed collaborative programs with experts within NU, other universities, and biotech and pharmaceutical industries. Karger's ability to bring together and work with world-class scientists and manage large research groups and budgets reflects his enthusiasm and dedication to the advancement of science.
Proteomics, Nucleic Acids, and Diagnostic Studies
In the mid-1980s, Karger turned his attention to capillary electrophoresis (CE), a high-resolution electrophoretic method introduced by Jim Jorgenson of the University of North Carolina at Chapel Hill, that had the potential for replacing slab-gel electrophoresis. Karger's initial CE studies showed very impressive separations of peptides and proteins (12) and other biologicals. Furthermore, his laboratory was the first to show the high resolution potential of CE for the separation of oligonucleotides, which led to his efforts in DNA sequencing (13).
Based on these and other results in the late 1980s, Karger was awarded one of the first National Institute of Health (NIH) grants for high-resolution CE separation of DNA in support of the Human Genome Project. The group's first major contribution was the development of capillaries filled with noncrosslinked matrices, which were found to be much more stable and to have higher efficiencies than crosslinked gels (14). An important finding by his laboratory was that these polymer matrices could be easily removed and replaced with fresh matrix, while maintaining high efficiency separations (15). These capillaries allowed for 24 h per day automated operation and demonstrated a viable approach for high-throughput sequencing.
From a fundamental understanding of migration of oligonucleotides through a polymer matrix, Karger's group was the first to show DNA sequencing of more than 1000 bases per run by CE using replaceable linear polyacrylamide (Figure 4) (16); several years later, the group reached 1300 bases (17). Interestingly, because of restricted diffusion of oligonucleotides, the efficiency of the CE columns with noncrosslinked linear polyacrylamide reached an astonishing 10 million theoretical plates! The linear polyacrylamide matrix was licensed to Beckman Coulter who then supplied it to Molecular Dynamics to sequence a fairly large portion of the human genome.

Figure 4: An example of rapid DNA sequence analysis of over 1000 bases for each run by capillary electrophoresis (CE) with replaceable polyacrylamide solutions and multiwavelength fluorescence emission. Sequencing labeling chemistries were performed on ssM13mp18DNA. The number of resolvable bases is given on the upper portion of each of the eight panels. Adapted and reproduced with permission from reference 16, copyright American Chemical Society.
With the completion of the Human Genome Project, Karger returned to LC–MS, focusing on protein analysis and proteomics. He had already produced a significant amount of research on CE–MS coupling (18), including CE–matrix-assisted laser desorption-ionization (MALDI)–time of flight (TOF)-MS coupling (19). In addition, he showed the potential of coupling microfluidic separation devices to MS (20), which are now commercially available. Karger turned his attention to improving sensitivity and lowering the detection limits of LC–MS of peptides and proteins. Because very low mobile phase flow rates (low nanoliters per minute) increased ionization efficiency in electrospray ionization with mass spectrometry (ESI–MS), his group introduced 20-µm i.d. polymer monolithic columns for high performance separations with low-attomole detection (21).
To further increase performance, Karger's group introduced 10-µm i.d. porous-layer open tubular (PLOT) columns for high-resolution, ultratrace LC–MS analysis of proteomic and protein samples (22). Analogous to GC capillary columns, such columns can generate a large number of plates, since the column lengths are typically 3–5 m. Figure 5 shows a one-dimensional (1D) proteomic separation with a peak capacity over 400. Continual separation improvements were made, including two-dimensional (2D) separations using a triphasic capillary containing reversed-phase trapping, ion-exchange chromatography (IEC), and then reversed-phase trapping again, prior to the PLOT column (23). Low-level detection for disease tissue analysis using PLOT LC–MS continues to the present day in Karger's laboratory (24). Karger now has a number of collaborative projects with clinicians to study diseases such as breast cancer (25), in the hope of finding protein biomarkers and signatures that can ultimately be useful diagnostic tools.
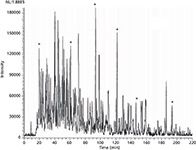
Figure 5: This figure shows the results of ultratrace analysis of a nanogram-quantity of tryptic digest using a 4.2-m long porous-layer open-tubular poly(styreneâdivinylbenzene) capillary column using a nano-LCâESIâMS system. The peak capacity of the column is about 400. Adapted and reproduced with permission from reference 22, copyright American Chemical Society.
Using LC–MS, his group then turned to protein characterization of more complex, biological systems such as the phosphorylation of epidermal growth factor, an important receptor (26). With the introduction of electron transfer dissociation-collision-induced dissociation (ETD-CID) MS, his laboratory used this LC–MS approach for disulfide linkage identification (27), including complicated cysteine knot structures (28), a study that was reported just last year. ETD-CID was also used for the LC–MS discrimination of isoaspartic acid from aspartic acid (29). All of these studies were accomplished with bottom-up approaches involving the separation of peptides.
Karger has also done work in middle-down LC–MS to determine post-translational modifications, for example, phosphorylation on long peptide chains (30). Furthermore, glycan analysis of biopharmaceuticals has been another area of interest; CE with laser-induced fluorescence detection is used for high-resolution separation and low-level detection of derivatized glycans (31). Finally, LC–MS using a microfluidic device containing porous graphitized carbon black and negative ESI for enhanced glycan structure analysis appeared last year (32).
It is indeed amazing that Karger can be interested and productive on so many fronts at this stage of his career, in addition to his teaching and administration duties as Director of the Barnett Institute of Chemical and Biological Analysis.
Barnett Institute of Chemical and Biological Analysis
In 1973, Karger assisted the College of Criminal Justice at NU, under the leadership of the late Dean Norman Rosenblatt, to obtain a grant from the US Department of Justice to establish graduate-level programs of importance in criminal justice. Through this grant, Karger was appointed director of research and given the opportunity to establish a multidisciplinary program housed within the Department of Chemistry. In 1973, the Institute of Chemical Analysis and Forensic Science was born with Karger as the founding director, a position he holds to this day.
In 1983, Louis Barnett, a 1943 alumnus of NU, provided an endowed naming gift to the Institute to help expand its coverage of research activities. With Barnett's support and other sources of funding, the newly named Barnett Institute of Chemical and Biological Analysis evolved over the years; its current mission is to develop and apply new technologies for basic and clinical biological research, and for the characterization of protein therapeutics.
Since its inception, the Institute has trained nearly 350 scientists and graduate students, published over 1000 papers, and issued close to 80 patents. A very strong program in the development and application of separations coupled to mass spectrometry is now in place. Furthermore, the Institute helped to breathe life into five companies, the newest being BioAnalytix, a high-level protein characterization company that supports the biotech industry. In 2013, the Institute announced a technology alliance partnership with Thermo Fisher Scientific in Karger's laboratory which will focus on new MS instrumentation for protein characterization, trace analysis of biomarkers in proteomic samples, and for analyzing biosimilar drugs.
Modern Approach to Bioanalytical Separations
High-resolution separations of biological samples consist of LC–MS or CE–MS with specially designed interfaces. Mass spectroscopy is not only the best detection approach for chemical structure elucidation of trace quantities of complex, large molecules, but the MS technique itself also adds an additional dimension of high-resolution separation. Although there are some mass spectrometrists who consider separations as simply a convenient way of preparing clean samples, most will admit that the separation step is key to the success of an analysis.
According to Karger, analytical chemistry should be approached from a holistic point of view; that is to say, the problem at hand should be treated in its entirety, rather than focusing solely on individual steps of the analysis. Therefore, as one parameter of the separation is optimized, its effect on all the other steps should be considered. With this in mind, significant progress can be made by developing the separation in tandem with the detection system. This strategy calls for careful planning, a willingness to change direction if needed, and, most importantly, close collaboration between the separation scientist and mass spectrometrist. Furthermore, both groups of scientists should try to stay abreast of each other's literature and have a working knowledge of the principles, advantages, and limitations of the disciplines. This "big picture" approach, although time-consuming, should be practiced and applied by researchers when faced with complex analytical problems. With close to 350 publications, more than 40 patents, and approximately 50 awards and honors (see Table I) to his credit, Karger must be doing something right!
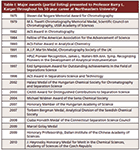
Table I: Major awards (partial listing) presented to Professor Barry L. Karger throughout his 50-year career at Northeastern University
50 Years at the Helm and Still Going Strong
Those of us that know Karger realize that he is a voracious reader, staying abreast of new technologies, not only in areas within his scope of interest, but also in associated disciplines as well. He has encouraged and trained first-generation chromatographers and second-generation bioanalytical chemists, representing a remarkable 50-year span. Karger has been directly responsible for mentoring 90 PhD students, working with more than 70 postdoctoral fellows and visiting scientists, and managing 20 staff scientists at the Barnett Institute, many of whom have either had or have gone on to have distinguishing scientific careers (see Table II for a partial list).

Table II: Partial list of prominent scientists who either collaborated with or trained under Barry L. Karger during the past 50 years at Northeastern University
Although some of his former students and many of his colleagues have retired or are close to retirement, at 74 Karger is almost as active as he was 50 years ago.
Karger looks upon each postdoc and graduate student as his most satisfying accomplishment when they leave his laboratory and venture forth as productive scientists. He always mentions how proud he is of the success of all of his students; and naturally we are proud of him. Congratulations Barry!
References
(1) B.L. Karger and L.B. Rogers, Anal. Chem. 33, 1165 (1961).
(2) H. Barth, E. Dallmeier, and B.L. Karger, Anal. Chem. 44, 1726 (1972).
(3) H. Barth, E. Dallmeier, G. Courtois, H.E. Keller, and B.L. Karger, J. Chromatogr. 83, 289 (1973).
(4) B.L. Karger, L.R. Snyder, and C. Horváth, An Introduction to Separation Science, (Interscience, New York, USA, 1973).
(5) B.L. Karger, L.R. Snyder, and C. Eon, J. Chromatogr. 125, 71 (1976).
(6) B.L. Karger, J.R. Gant, A. Hartkopf, and P.H. Weiner, J. Chromatogr. 128, 65 (1976).
(7) N. Tanaka, H. Goodell, and B.L. Karger, J. Chromatogr. 158, 233 (1978).
(8) B.L. Karger, J.N. LePage, and N. Tanaka, in HPLC-Advances and Perspectives, C. Horváth, Ed., (Academic Press, New York, USA, Vol. 1, 1980).
(9) W. Lindner, J.N. LePage, G. Davies, D.E. Seitz, and B.L. Karger, J. Chromatogr. 185, 323 (1979).
(10) K. Benedek, S. Dong, and B.L. Karger, J. Chromatogr. 317, 227 (1984).
(11) N.T. Miller, B. Feibush, and B.L. Karger, J. Chromatogr. 316, 519 (1985).
(12) A.S. Cohen, S.C. Terabe, J.A. Smith, and B.L. Karger, Anal. Chem. 59, 1021 (1987).
(13) A.S. Cohen, D.R. Najarian, A. Paulus, A. Guttman, J.A. Smith, and B.L. Karger, Proc. Natl. Acad. Sci. 85, 9660 (1989).
(14) D.N. Heiger, A.S. Cohen, and B.L. Karger, J. Chromatogr. 516, 33 (1990).
(15) M.C. Ruiz-Martinez, J. Berka, A. Belenkii, F. Foret, A.W Miller, and B.L. Karger, Anal. Chem. 65, 2851 (1993).
(16) E. Carrilho, M. Ruiz-Martinez, J. Berka, I. Smirnov, W. Goetzinger, F. Foret, A.W. Miller, D. Brady, and B.L. Karger, Anal. Chem. 68, 3305–3313 (1996).
(17) H. Zhou, A.W. Miller, Z. Sosic, A.E. Barron, B. Buchholz, L. Kotler, and B.L. Karger, Anal. Chem. 72, 1045 (2000).
(18) B. Zhang, F. Foret, and B.L. Karger, Anal. Chem. 72, 1015–1022 (2000).
(19) T. Rejtar, P. Hu, P. Juhasz, J.M. Campbell, M.L. Vestal, J. Preisler, and B.L. Karger, J. Proteome Res. 1, 171–179 (2002).
(20) Q. Xue, F. Foret, Y.M. Dunayevskiy, P.M. Zavracky, N.E. McGruer, and B.L. Karger, Anal. Chem. 69, 426–430 (1997).
(21) A. Ivanov, L. Zang, and B.L. Karger, Anal. Chem. 75(20), 5306–5316 (2003).
(22) G. Yue, Q. Luo, J. Zhang, S. Wu, and B.L. Karger, Anal. Chem. 79(3), 938–946 (2007).
(23) Q. Luo, Y. Gu, S. Wu, T. Rejtar, and B.L. Karger, Electrophoresis 29, 1604–1611 (2008).
(24) D. Wang, M. Hincapie, T. Rejtar, and B.L. Karger, Anal. Chem. 83, 2029–2037, (2011).
(25) M. Imielinski, S. Cha, T. Rejtar, E.A. Richardson, B.L. Karger, and D. Sgroi, Mol. Cell Proteomics 11(1), (2012).
(26) S.L. Wu, J. Kim, R. Bandle, L. Liotta, E. Petricoin, and B.L. Karger, Mol. Cell. 5, 1610–1627 (2006).
(27) S. Wu, H. Jiang, W. Hancock, and B.L. Karger, Anal. Chem. 82, 5296–5303 (2010).
(28) W. Ni, M. Lin, P. Salinas, P. Savickas, S.L. Wu, and B.L. Karger, J. Am Soc Mass Spectrom. 24(1), 125–33 (2013).
(29) W. Ni, S. Dai, B.L. Karger, and Z. Zhou, Anal. Chem. 82, 7485–7491 (2010).
(30) J. Zhang, S. Wu, J. Kim, and B.L. Karger, J. Chromatogr. A. 1154, 295–307 (2007).
(31) Z. Szabo, A. Guttman, J. Bones, and B.L. Karger, Anal. Chem. 83, 5329–5336 (2011).
(32) W. Ni, J. Bones and B.L. Karger, Anal. Chem. 85(6), 3127–35 (2013).
Howard G. Barth retired from the DuPont Company as senior research associate, where he was involved with SEC, HPLC, and polymer characterization. He was appointed associate editor of the Journal of Applied Polymer Science, and also book series editor of Springer's Laboratory Manuals in Polymer Science. He was founding editor and editorinchief of the International Journal of Polymer Analysis and Characterization, and was chair for many years of the International Symposium on Polymer Analysis and Characterization. He works as a consultant in chromatography, analytical chemistry, and technical editing and writing. Direct correspondence to: howardbarth@gmail.com

Howard G. Barth

Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








