Probe Electrospray Ionization-Mass Spectrometry and Isotope Dilution Approaches for Ultrafast Measurement of Metformin
Metformin (MtF) is a first-line treatment used for type 2 diabetes for which lactic acidosis (LA) is a serious and frequent complication induced by or associated with elevated MtF plasma concentrations. In this study, a probe electrospray ionization coupled to mass spectrometry (PESI-MS) method for ultra-rapid determination of MtF in plasma by isotope dilution was developed, with no sample preparation or calibration curve building. Satisfactory results of accuracy and precision (min–max: -16.4–15.8% and 1.0–17.2%) were obtained. In a representative group of patients, almost identical results were obtained when comparing liquid chromatography–diode array detection (LC–DAD) and LC–tandem mass spectrometry (MS/MS) to PESI-MS (r2 > 0.99).
Metformin (MtF) is an oral antihyperglycaemic agent widely used for type 2 diabetes (1). Although hypoglycemia is not an expected toxic effect of metformin, lactic acidosis (LA) is of great concern in overdosed patients. Metformin is excreted unchanged in urine and could accumulate in the case of acute kidney injury. A metformin plasma concentration > 9.9 mg/L is strongly associated with the presence of LA (threshold value with a specificity of 92.6%) (2). Consequently, metformin accumulation must be rapidly identified in patients admitted to emergency or intensive care units. The confirmation of an elevated metformin plasma concentration is essential to help clinicians establish the diagnosis of LA and to initiate an adequate treatment. The evolution of LA depends on the early initiation of renal replacement therapy (RRT).
What is PESI?
Probe electrospray ionization (PESI) can be seen as a miniaturization of electrospray (ESI), where the capillary of the nanoelectrospray converges on a solid needle with a tip of several hundred nanometres. This probe is coupled directly to a mass spectrometer (MS) without the need for chromatographic separation, allowing ultrafast analysis with minimal sample preparation. The principles of PESI have been extensively detailed by Hiraoka et al. (3), but it can be seen as follows: a probe needle repeatedly moves down (“dives” into the sample) and moves up (to the position when ionization occurs), and consequently the compounds are periodically ionized and their resultant ions are pushed into the tandem mass spectrometry (MS/MS) system. PESI-MS has already been successfully applied to the analysis of biological samples (4,5).
A PESI/MS-MS Method Dedicated to the Measurement of Metformin: An Original Approach with Isotope Dilution Protocol
The principle of the method can be summarized as follows: A 10 µL measure of plasma was mixed with 1000 µL of a 10 mM 1:1 (v/v) ethanol–ammonium formate buffer (see Figure 1). Next, a solution of deuterated internal standard of MtF (MtF-D6) at 5 mg/L was added. Finally, 10 µL of this mixture was spiked on the dedicated plastic sample plate and placed into the PESI ion source (Shimadzu).
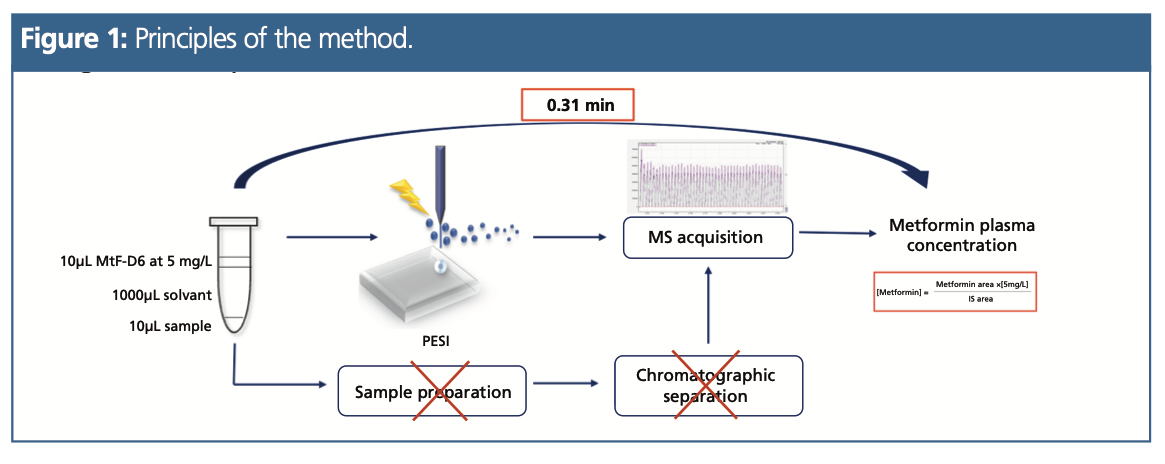
A Shimadzu 8060 triple quadrupole mass spectrometer was used in positive ionization mode. The vertical movement of the needle was repeated with a frequency of 3.1 Hz (184/min) for a run-time of 0.31 min.
MtF and MtF-D6 were detected with multiple reaction monitoring (MRM). The selected transitions were m/z 130.15 > 71.10 for quantitation, and m/z 130.15 > 60.05 as qualifier ion for metformin. The transitions were m/z 135.95 > 77.15 and m/z 135.95 > 60.05 for MtF-D6.
To apply an isotope dilution protocol, the main MS parameters were optimized to obtain the same intensity for the m/z 130.15 > 71.10 (MtF) and m/z 135.95 > 77.15 (MtF-D6) transitions. According to the isotope dilution principle, each concentration level was determined using the ratio between the peak area of MtF and MtF-D6 multiplied by its concentration, which was fixed at 5 mg/L.
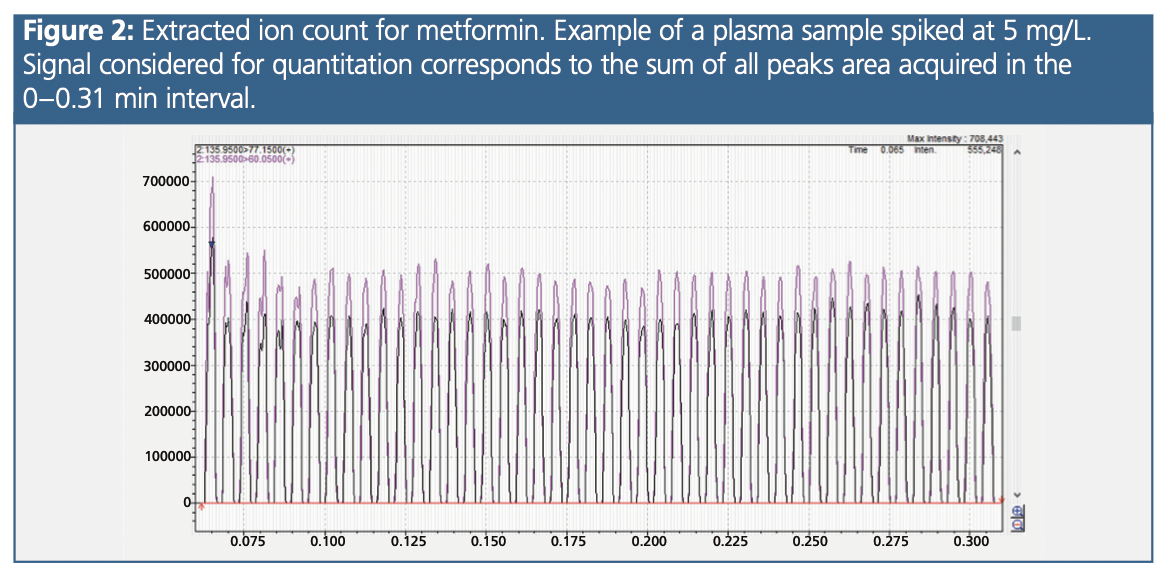
Five concentration levels (0.5, 2.5, 5, 10, and 50 mg/L) were considered for the validation of the method. The inter‑day precision (coefficient of variation, CV) and accuracy (bias) were determined for each concentration level using the isotope dilution approach. The intra-day precision and accuracy were assessed for each level (n = 6 for the same day). The method’s validation parameters are summarized in Table 1.
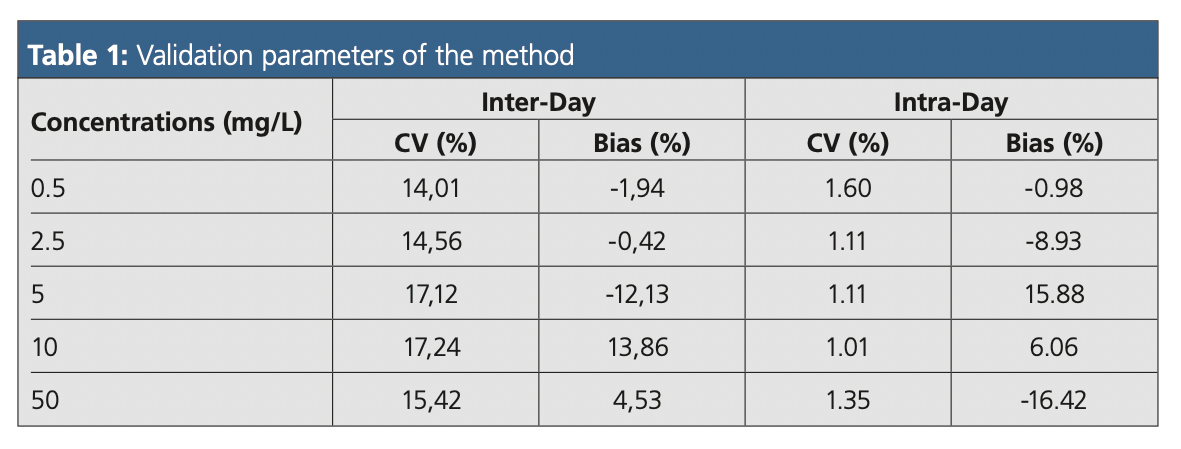
Results
A typical extracted ion count for Mtf is presented in Figure 2. Concerning the matrix effects, we observed an average enhancement of 57% between the metformin signal obtained in the six different plasma samples and those obtained in the purified water samples. However, this matrix effect was fully corrected by the internal standard (bias of 1% between plasma and water after correction).
A study exploring the specificity with a solution of 52 potential co-medications reported satisfactory results. The ratio between the peak areas measured in the blank and the peak areas in the lower limit of quantitation (LLOQ) samples were systematically less than 5% for metformin (from 2.5 to 4.7%) and metformin-D6 (from 0.13 to 1.67%).
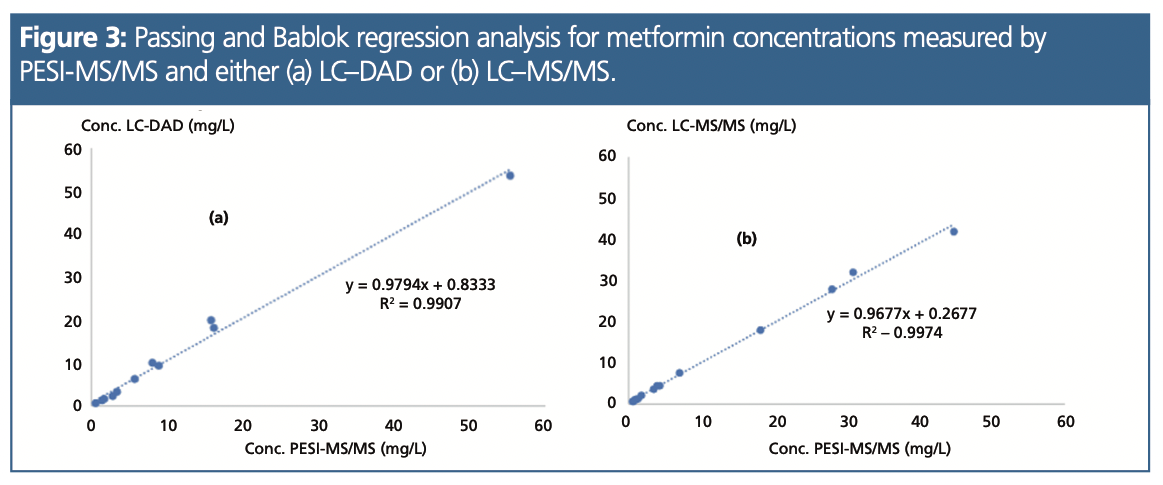
The concentrations of metformin were measured with PESI-MS and two other analytical methods (liquid chromatography [LC]–MS/MS or LC–diode array detection [DAD]) in 29 patients, from which samples were obtained from the routine activity. Figure 3 reports the regression analyses between PESI-MS and these two methods. In this representative group of patients (55% with a concentration <5 mg/L, 38% with a concentration >5 mg/L, and 7% not detected), excellent agreements were observed either with LC–DAD (r2 = 0.99; n = 11) or LC–MS/MS (r2 = 0.99; n =16).
Discussion and Perspectives
When combined with an isotope dilution protocol that requires no calibration curve building, the PESI-MS approach offered the best possibility to design specific, sensitive, accurate, and ultra-fast solutions. This study presents a method that allows an accurate determination of metformin in about 30 s, and will be helpful in a core laboratory when rapid diagnosis of LA is needed. In a clinical emergency setting, we propose analyzing a metformin control quality sample spiked at 5 mg/L together with the patient sample. When taking into account all the manual steps (dilution and spiking of the sample on the plate), it is estimated that less than 5 min are needed to measure metformin in a patient.
With this PESI-MS approach, there is no sample extraction and no column separation process. No particular skill in LC–MS is needed, as the operator simply dilutes the plasma in an ethanol–formate buffer, adds the IS solution, and then sets the sample plate into the PESI source. It is also not necessary to dedicate a MS system to the PESI technology. In fact, the PESI source can be installed and removed in front of the MS system and is interchangeable with a classical ESI source without shutting down the system.
There are on-going studies to develop similar approaches for an ultra‑fast measurement of other classes of drugs useful in a core lab (for example, benzodiazepines, antiepileptics, and illicit drugs).
References
- Graham, G. G.; Punt, J.; Arora, M.; et al. Clinical Pharmacokinetics of Metformin. Clinical Pharmacokinetics2011, 50 (2), 81–98. DOI: 10.2165/11534750-000000000-00000
- Bennis, Y.; Bodeau, S.; Batteux, B.; et al. A Study of Associations Between Plasma Metformin Concentration, Lactic Acidosis, and Mortality in an Emergency Hospitalization Context. Critical Care Medicine 2020, 48, 1194–1202. DOI: 10.1097/CCM.0000000000004589
- Hiraoka, K.; Nishidate, K.; Mori, K.; Asakawa, D.; Suzuki, S. Development of Probe Electrospray Using a Solid Needle. Rapid Communications in Mass Spectrometry 2007, 21, 3139–3144. DOI: 10.1002/rcm.3201
- Usui, K.; Kobayashi, H.; Fujita, Y.; et al. An Ultra-Rapid Drug Screening Method for Acetaminophen in Blood Serum Based on Probe Electrospray Ionization-Tandem Mass Spectrometry. Journal of Food and Drug Analysis 2019, 27, 786–792. DOI: 10.1016/j.jfda.2019.02.001
- Usui, K.; Minami, E.; Fujita, Y.; et al. Application of Probe Electrospray Ionization-Tandem Mass Spectrometry to Ultra-Rapid Determination of Glufosinate and Glyphosate in Human Serum. Journal of Pharmaceutical and Biomedical Analysis 2019, 174, 175–181. DOI: 10.1016/j.jpba.2019.05.040
Pauline Griffeuille is a PharmD, intern in pharmacy and PhD student. She obtained a master’s in analytical chemistry in 2020.
Sylvain Dulaurent is an engineer in analytical chemistry.
Franck Saint-Marcoux is head of the unit at Limoges University Hospital.

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)















