Perspectives and Pitfalls in Potency Testing of Cannabinoids by High Performance Liquid Chromatography (HPLC)
Cannabis products have been recently legalized in many countries for recreational or medicinal use. Therefore, rigorous analytical methods to test the potency of samples is required prior to commercialization. In addition, growing interest in the properties of minor cannabinoids has increased the demand for high-throughput methods that can separate the largest number of compounds in the shortest amount of time. High performance liquid chromatography (HPLC) is emerging as the preferred analytical method for potency testing of cannabinoids, but more fundamental work is needed to solve critical issues and contribute to advancing knowledge.
Cannabis sativa L. is one of the most discussed plants in the world. On the one hand, it is popular from a toxicological perspective because marijuana (a greenish-gray mixture obtained from dried flowers of the plant) represents the most common illicit drug of abuse in the world. On the other hand, therapeutic properties of C. sativa L. have been recognized for millennia, especially for pain and appetite modulation or regulation of other biological functions, such as sleep, memory, and nausea.
Among the many classes of bioactive compounds contained in C. sativa L., the family of phytocannabinoids is the most investigated because it is responsible for both the therapeutic and psychotropic effects of C. sativa L. Cannabinoids are C21 (or C22 for the carboxylated form) terpenophenolic compounds that are able to interact with the endocannabinoid system that is present in the human body (1). Phytocannabinoids are produced naturally in the acid form by the plant, and can be transformed easily into their correspondent decarboxylated metabolites after exposure to heat or light, or both. To date, more than 100 cannabinoids are known, but their number is expected to grow. Among these, the most popular are Δ9-trans-tetrahydrocannabinol (Δ9-THC), which is mainly responsible for psychotropic effects, and cannabidiol (CBD), which is less prone to interact with the endocannabinoid system and therefore not mind-altering (2). CBD is attracting great interest for its potential pharmacological properties. Clinical research is being conducted to study its beneficial effects in the treatment of epilepsy or other neurological disorders (including Parkinson’s disease and Tourette’s syndrome) and in appetite stimulation in oncology and HIV patients (3).
In the last several years, several countries, and many states in the United States, have legalized cannabis for medical purposes or marijuana for recreational use (or even both). These new regulations and laws have led to a revolution in the cannabis industry. As a result, production has tremendously increased, and that has translated into an increased need to develop reliable quality control (QC) methods to identify cannabinoids and test the potency of cannabis products and derivatives. Potency testing refers to the quantification of the main cannabinoids contained in a sample in terms of weight percentage. This information is fundamental for the accurate labeling of cannabis products for both medical and recreational use. To date, potency testing has required the quantification of only five cannabinoids, namely a) Δ9-THC; b) CBD; c) tetrahydrocannabinolic acid (THCA); d) cannabidiolic acid (CBDA); and e) cannabinol (CBN). However, other cannabinoids have been recently added to the list of those that should be quantified, including Δ8-THC, cannabigerol (CBG), cannabichromene (CBC), and cannabidivarin (CBDV). Because new cannabinoids are continuously discovered, it is expected that the list of compounds for potency testing will need to be expanded again.
Because cannabinoids were separated initially and identified exclusively through gas chromatography with flame ionization detection (GC-FID), GC-FID is still the method indicated by European Union (EU) regulatory agencies for the quantitative determination of Δ9-THC (4). However, GC has some limitations. First, the high temperatures employed at the injection port lead to the decarboxylation of the acid cannabinoids to their correspondent neutral forms, which can occur to different extents depending on experimental conditions. To prevent this process from occurring, proper derivation is needed, but the method must be validated (5). In addition, high temperatures may also favor isomerization or oxidation reactions. For these reasons, in the last few years, there has been an increased interest in alternative separation techniques, among which high performance liquid chromatography (HPLC) has become the preferred option. This paper discusses the latest achievements and pitfalls in potency testing of cannabinoids, mainly by HPLC. Perspectives and research opportunities in this field are also considered.
Potency Testing of Cannabinoids by HPLC
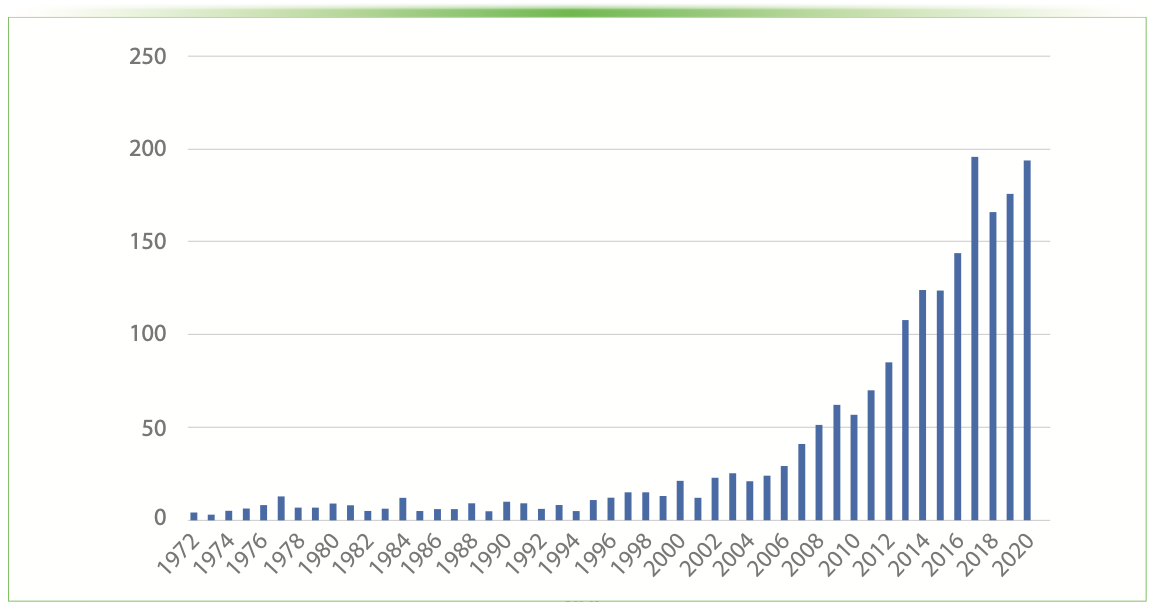
The great versatility of HPLC, coupled with the absence of derivatization steps or sample pretreatment before injection, explains why this technique has become the gold standard for potency testing (6,7) and for characterizing cannabinoids. The results of a simple Scopus search based on the keywords “cannabinoids” and “liquid chromatography” show that the number of papers published on this topic (Figure 1) has dramatically increased in the last years.
FIGURE 1: The number of papers published in the literature on the search terms “cannabinoids” and “liquid chromatography” (from Scopus database, June 2021).
Cannabinoids are mainly separated under reversed-phase LC (RPLC) conditions with C18 stationary phases. Resolution of species with very similar chemical structures (such as the Δ9-THC/Δ8-THC pair) can be particularly challenging. High-efficiency stationary phases based on superficially porous particles (SPPs) and sub-2-μm fully porous particles (FPPs) have been employed in these cases (6). There is increasing interest in high-throughput separation of cannabinoids by HPLC that goes hand-in-hand with the need for fundamental studies on the retention of cannabinoids, especially to increase the resolution of critical pairs and shorten analysis times. To this end, novel sorbents need to be explored, including mixed-mode stationary phases. On the other hand, supercritical fluid chromatography (SFC) seems to be suitable for the separation of cannabinoids, especially chiral ones. Therefore, it can be expected that application of SFC in the field will increase greatly in the near future (6–8).
Because new cannabinoids are continuously discovered, even potency testing needs to be updated. The first important issue is the lack of reference methods. In addition, not all discovered cannabinoids are available as certified reference material. Therefore, in case of need, they must be either synthesized or isolated from complex samples.
Enantioseparation of Cannabinoids
Recently, scientists have started giving attention to another important aspect of cannabinoids: their chirality. Indeed, it is known that many of these molecules are chiral, but little (or even nothing) is known about the biological activity of the two enantiomers (9,10). The first important difficulty is that the production of natural chiral cannabinoids could be hidden by racemization occurring after sample preparation (exposure of samples to heat and light). On the other hand, especially if the two enantiomers have different biological activity, information on enantiomeric purity and stereostability should be important for regulatory purposes.
A quick look at the literature reveals that just a few papers have been published on the chirality of cannabinoids. When the search is restricted to the separation of chiral cannabinoids via HPLC, no more than a dozen of works are found, mostly regarding the separation of synthetic cannabinoids. Those few papers on the separation of chiral phytocannabinoids date back to more than 20 years ago (11–13). In these studies, single pairs of Δ8-THC and CBD were separated on a cellulose-based chiral stationary phase (CSP) under normal-phase LC (NPLC) conditions. It is only recently that various companies have published technical notes or examples showing the separation of Δ8-THC and Δ9-THC enantiomers or their degradation products, again by using polysaccharide CSPs under NPLC conditions (14,15). In our department, we are currently working on the investigation of retention behavior of cannabinoids on polysaccharide CSPs in collaboration with Chiral Technologies (manuscripts in preparation).
Recent advances in both the chemistry of CSPs and the geometrical characteristics of particles used as base material in chiral LC (either sub-2-μm FPPs or SPPs) have expanded possibilities in this field (16,17). As an example, a recent work co-authored by some of us has shown the great potential of the so-called inverted chirality columns approach (ICCA) for the enantioseparation of chiral cannabinoids (9). This method requires the use of CSPs with chiral selectors of opposed configurations (Whelk-O1 CSPs). Such an approach allows the inversion of the elution order of enantiomers depending on the “chirality” of the column employed, which is particularly advantageous in the absence of reference standards. In the specific case reported in reference (9), the authors operated (S,S)- and (R,R)-Whelk-O1 columns in SFC conditions for the separation of an extract of medicinal cannabis. They found that the enantiomeric excess of (-)-Δ9-THC is not 100% as expected, but 99.73%. Apart from (-)-Δ9-THC (that has been classically considered to be naturally produced by the plant), a small but not negligible percentage of the corresponding (+)-enantiomer (0.13%) was found.
Conclusions
There is an apparent contradiction between the popularity of C. sativa L. and the relatively scarce research on fundamental aspects of analytical methods for the regulation and quality control of its products. Chemical characterization and classification of cannabis is particularly challenging because novel phytocannabinoids are continuously being discovered. HPLC with UV detection is emerging as the gold standard for routine potency testing analysis, replacing GC-FID methods. The use of state-of-the-art columns packed with either SPPs or sub-2-μm FPPs makes it possible to separate a large number of cannabinoids, even if more fundamental work is needed to improve the baseline resolution of critical pairs of species almost co-eluted under the experimental conditions tested to date. Mixed-mode sorbents could help to solve this issue.
Another aspect that is catching on is fundamental research on the chirality of cannabinoids. Recent advances in column technology and particle manufacturing will bring novel opportunities for detecting minor chiral impurities, and these developments will also help us understand their potential benefits or harmfulness for human health.
References
(1) C.E. Turner, M.A. Elsohly, and E.G. Boeren, J. Nat. Prod. 43, 169–234 (1980).
(2) G. Appendino, G. Chianese, and O. Tagliatela-Scafati, Curr. Med. Chem. 18, 1085–1099 (2011).
(3) E.M. Williamson and F.J. Evans, Drugs 60, 1303–1314 (2000).
(4) Reg. (EU) 639/2014 Annex III as amended by Reg. (EU) 2017/1155, Union method for the quantitative determination of Delta-9-tetrahydrocannabinol content in hemp varieties (2017).
(5) F.E. Dussy, C. Hamberg, M. Luginbühl, T. Schwerzmann, and T.A. Briellmann, Forensic Sci. Int. 149, 3–10 (2005).
(6) S. Felletti, C. De Luca, A. Buratti, D. Bozza, A. Cerrato, A.L. Capriotti, A. Laganà, A. Cavazzini, and M. Catani, J. Chromatogr. A. 1651, 462304 (2021).
(7) C. Denicola and J.M. Barendt, “Cannabinoid isolation models utilizing immobilized chiral stationary phases and SFC,” paper presented at 2018 Emerald Conference, San Diego, California, 2018.
(8) C. Denicola and J.M. Barendt, “Streamlined cannabinoid and potency assays utilizing HPLC and SFC,” paper presented at Cannabis 2018 Conference, Oakland, California, 2018.
(9) G. Mazzoccanti, O.H. Ismail, I. D’Acquarica, C. Villani, C. Manzo, M. Wilcox, and A. Cavazzini, Chem. Commun. 53, 12262–12265 (2017).
(10) C.N. Filer, Cannabis Cannabinoid Res. 12, 1–4 (2021).
(11) S. Levin and S. Abu-Lafi, J. Chromatogr. A. 654, 53–64 (1993).
(12) S. Levin, M. Sterin, and S. Abu-Lafi, Chirality 7, 140–146 (1995).
(13) S. Abu-Lafi, M. Sterin, and S. Levin, J. Chromatogr. A. 679, 47–58 (1994).
(14) Chiral Technologies, “Separation of enantiomers of (+/-) Δ8-THC and (+/-) Δ9-THC,” Application Note, 2018.
(15) J. Williams, K. Calati, K. Hering, R.E. Franckowski, W.J. Umstead, and D.M. Iula, “HPLC Method to Differentiate Four THC Stereoisomers Formed from Δ9-THC Degradation: (6aR,9R)-Δ10-THC, (6aR,9S)-Δ10-THC, 9(R)-Δ6a,10a-THC, and 9(S)-Δ6a,10a-THC,” Application Note, Cayman Chemical, 2021.
(16) O.H. Ismail, S. Felletti, C. De Luca, L. Pasti, N. Marchetti, V. Costa, et al., Molecules 23, 2709 (2018).
(17) O.H. Ismail, M. Antonelli, A. Ciogli, C. Villani, A. Cavazzini, M. Catani, et al., J. Chromatogr. A. 1520, 91–102 (2017).
ABOUT THE AUTHORS
Martina Catani, University of Ferrara

Martina Catani, Simona Felletti, Alessandro Buratti, Chiara De Luca, and Alberto Cavazzini are with the Department Of Chemical, Pharmaceutical, and Agricultural Sciences at the University of Ferrara, in Ferrara, Italy. Direct correspondence to: martina.catani@unife.it.

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).














