A Quick and Accurate High Performance Liquid Chromatography (HPLC) Method to Determine the Amount of Trimethylamine in Fish Oil Softgels and Multivitamin Softgels Containing Fish Oil
Ocean fish cells contain trimethylamine oxide for osmoregulation. The odorless trimethylamine oxide reduces to trimethylamine by bacterial activity, resulting in a strong fishy odor. Therefore, it is important to find out whether trimethylamine levels in fish oil and multivitamin softgels containing fish oil will affect the quality of the finished products. A derivatization-based, reversed-phase high performance liquid chromatography (HPLC) method was developed using a Lichosphere 100 RP18 column, utilizing acetonitrile and water as the mobile phases. Experimental products were dissolved in ether, followed by a pH jump and buffer exchange, and finally derivatized with 9-fluorenylmethyl chloroformate. Our research has indicated that the level of trimethylamine in the products containing fish oil does not have a correlation with age and rancidity level of the products.
Fish oil contains polyunsaturated fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). These unsaturated fatty acids are susceptible to oxidation; as a result, fish oil rancidity is associated with the oxidation of the lipids (1). The fish oil industry uses anisidine values and peroxide testing for measuring the level of fish oil rancidity.
On the other hand, the trimethylamine (TMA) level is a measure of spoilage in fish and is believed to be the main contributor to fishy odor in fish (2) and humans (3,4).
To maintain fluid balance, marine animals need to fill their cells with amino acids and amines to compensate for the saltiness of seawater. Ocean fish use trimethylamine oxide (TMAO) for osmoregulation. Bacteria and enzymes reduce odorless TMAO into trimethylamine, which causes a strong fishy odor (Figure 1).
FIGURE 1: An illustration showing TMAO molecule reduction to TMA.

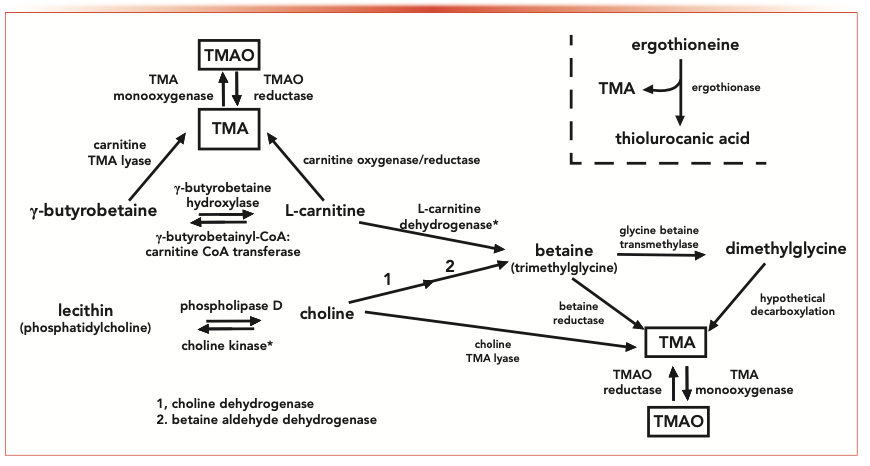
In the human body, TMA is produced from several metabolic pathways by human microbiota (Figure 2). A healthy human body can discharge the TMA; however, in some cases of TMA disorder disease, the TMA accumulates and results in unpleasant human body and urine odor (5).
FIGURE 2: An overview of TMA metabolic processes in the human body, from reference (3).
There are other studies relating fish oil rancidity with levels of aldehydes and secondary oxidants, which are not covered in this paper. However, there is no experimental evidence that correlates the level of TMA with fish oil rancidity. One of the objectives of this study was to develop an accurate method to quantitatively determine the level of TMA in fish oil softgels and multivitamin softgels containing fish oil. The other objective was to find the correlation between the level of TMA and rancidity of the fish oil-related products. In addition, products containing choline were investigated for the level of TMA and the product rancidity.
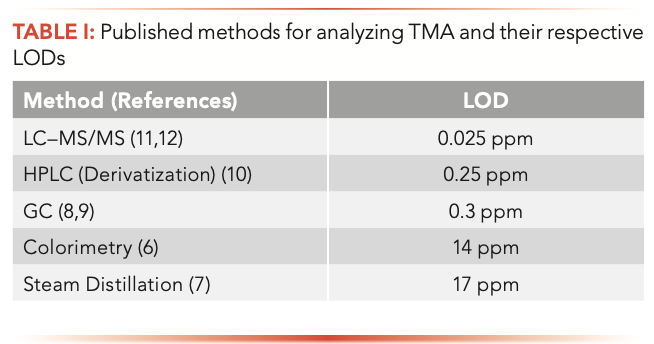
Different analytical methodologies have been used for analyzing TMA. In 1945, Dryer extracted TMA using toluene and analyzed it by colorimetric technic (6). In 1987, Malle and his colleagues utilized steam distillation by a deproteinization step with trichloroacetic acid and blocking of primary and secondary amines using formaldehyde at alkaline pH (7). In 1991, Fiddler and Oetjen used headspace gas chromatography (GC) and acid extraction for TMA measurement (8,9). Chafer used high performance liquid chromatography (HPLC) and a derivatization process to analyze trimethylamine in 2004 (10). In 2008 and 2017, respectively, Johnson and Yu used an electrospray ionization–tandem mass spectrometry (ESI–MS/MS) method for measuring TMA in urine using ethyl bromoacetate derivatization (11,12). Among all the analytical methodologies, LC–MS/MS had the lowest level of detection (LOD) (0.025 ppm), and the steam distillation method had the highest LOD at 17 ppm (Table I).
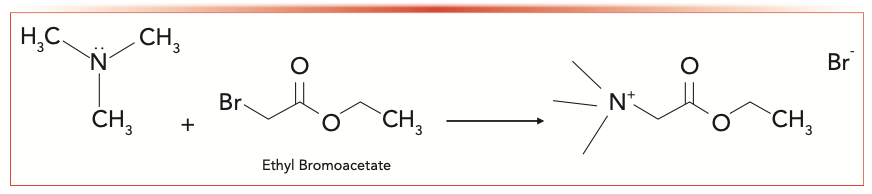
The HPLC-based derivatization method offers a low LOD and ease of instrument availability, which is one of the most widely accepted and used methods for TMA detection and quantitative analysis. There are two derivatization methods that have been reported in the literature, which are fluorenylmethyloxycarbonyl chloride (FMOC) (10) (Figure 3) and ethyl bromoacetate (13) (Figure 4). The FMOC was chosen in this research because there are two benzyl rings in FMOC, which have good UV absorption and strong response using a UV detector.
FIGURE 3: A trimethylamine derivatization reaction using FMOC.
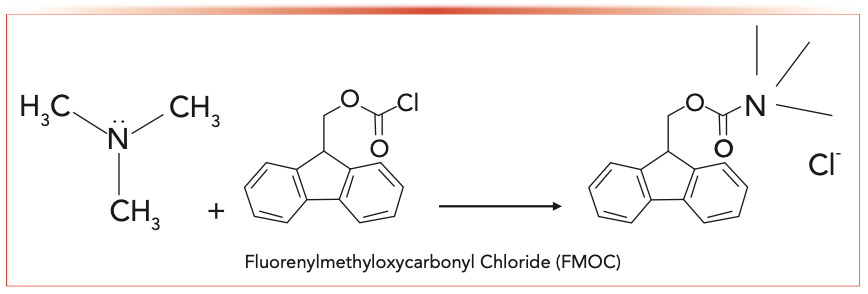
FIGURE 4: A trimethylamine derivatization reaction using ethyl bromoacetate.
The sample preparation is the next challenge to overcome. In this paper, TMA was successfully extracted from fish oil and multivitamin softgel products. TMA was then derivatized with FMOC to form TMA-FMOC, which has good UV detection. The derivatization kinetics were studied to give researchers options of performing online or offline derivatization to maximize method accuracy without sacrificing the ease of performing the tests.
Experimental
Materials
Trimethylamine hydrochloride salt, dimethyl ether, acetonitrile, disodium tetraborate, and 9-fluorenylmethyl chloroformate (FMOC) were purchased from Sigma Aldrich. A Milli-Q water system was used for water and buffer preparations.
The trimethylamine standard solution was prepared by adding 500 μL of standard solution to 1000 μL of FMOC solution (1 mg/mL) to make a 4 ppm trimethylamine-FMOC solution.
Fresh experimental fish oil softgels and fish oil-containing choline softgels were prepared at Pharmavite LLC. The samples were tested at the time of production before being packaged with the finished product packaging configuration and placed in an accelerated stability chamber at 40 oC/75 RH. The samples were tested after production (T0), 1 m, 2 m, and 3 m from the stability chamber, as well as samples stored at room temperature for more than 24 months (aged sample).
Multivitamin softgels containing fish oil (fresh and aged) products were prepared internally at Pharmavite LLC and fish oil raw material was purchased from C.I. Naturmega S.A. Other fish oil softgels were purchased from the market, manufactured by various commercial vendors.
Instruments
The analysis was carried out with an Alliance HPLC system from Waters Corporation with a 2996 photodiode array (PDA) detector. The detection signal was recorded, and the peak areas were quantified and processed with Empower software. The chromatographic column was Lichrospher 100 RP18 (125 mm x 4 mm, 5 μm particle size) from Supelco, and a linear gradient was used from 60:40 acetonitrile:water isocratic for 3.5 min and then to 70:30 acetonitrile:water in 10 min. The system flow rate was set at 1.0 mL/min. The injection volume was 10 μL. The detection wavelength for the PDA was set at 262 nm, and the analytical column was kept at 25 oC. Samples were kept at room temperature in the HPLC system.
Method of Extraction
To extract trimethylamine from products, 3 g of the chosen product were weighed in a centrifuge tube and dissolved in 10 mL of dimethyl ether. Then, TMA was extracted from dimethyl ether using 10 mL of 0.5 mM sodium tetraborate buffer at a pH of 9. The solution was centrifuged at 3000 rpm for 10 min, and then the aqueous layer was separated. The sodium tetraborate buffer extraction was repeated one more time and the aqueous layers were combined from the two extractions. Our study on multiple extractions proved that a two-time buffer extraction was enough. The samples were kept cool during the extraction process to minimize oxidation and evaporation.
Finally, 500 μL of combined aqueous solution was mixed with 1000 μL of FMOC solution (1 mg/mL) at room temperature for 2 h before being injected into the HPLC system. No agitation or vortexing is needed for full derivatization. The schematic extraction and derivatization processes are shown in Figure 5.
FIGURE 5: A schematic showing the TMA extraction process.
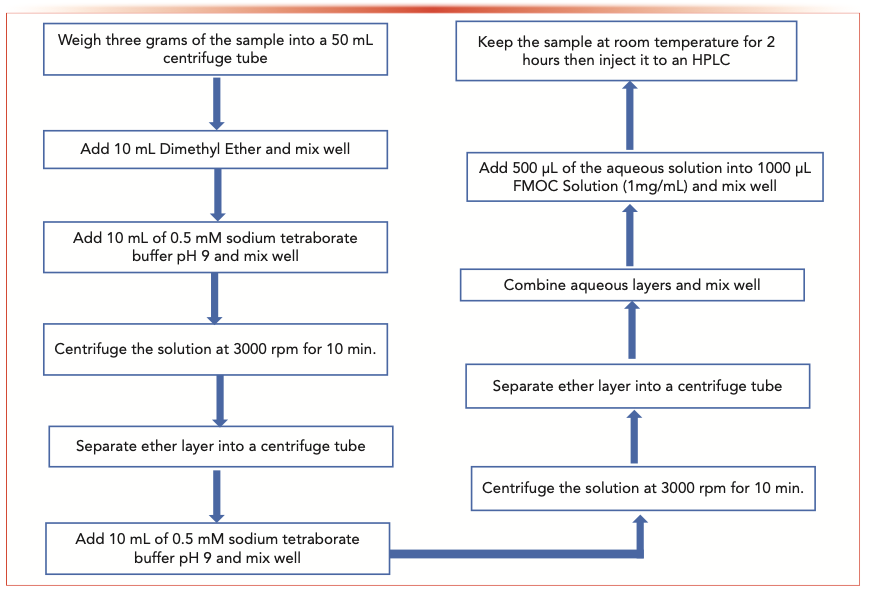
Results and Discussion
Standard Derivatization and Derivatization Kinetics
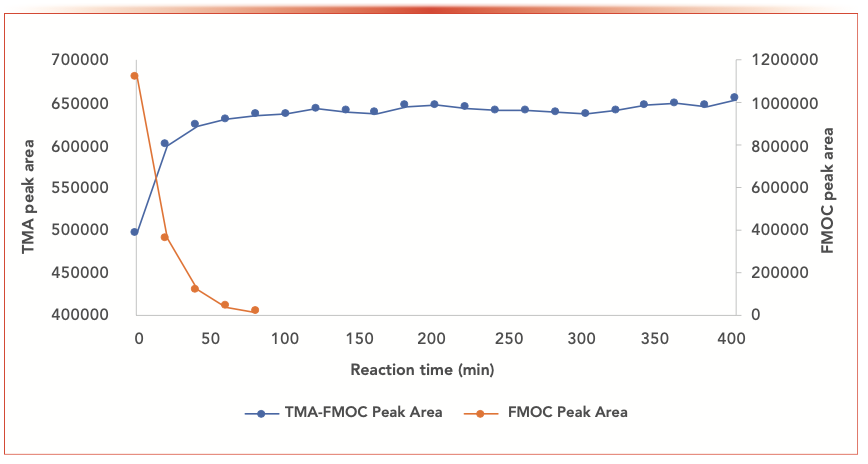
The FMOC-TMA derivatization kinetic study was performed by injecting the solution into the HPLC system in 20-min time intervals up to 400 min. The study shows the derivatization reaction reaches equilibrium after approximately 100 min and remains stable for 400 min (Figure 6). Therefore, all solutions were kept at room temperature for 2 h before being injected into the HPLC system.
FIGURE 6: A plot of the kinetics of TMA derivatization with FMOC.
Method Linearity and Accuracy
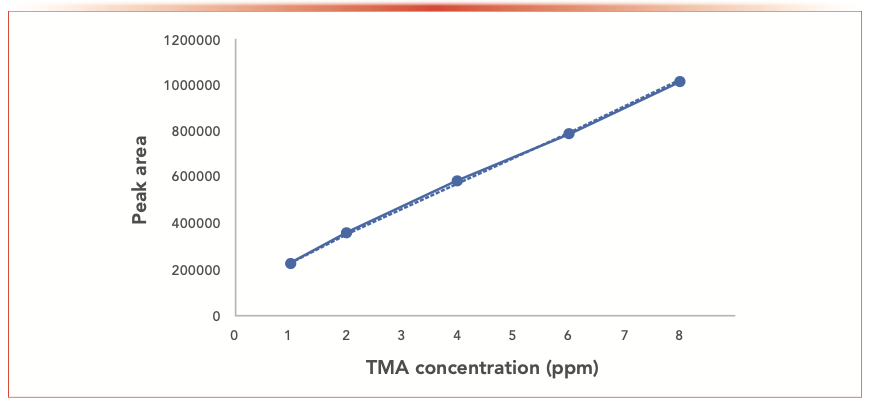
Good linearity was achieved in the range of 1–17 ppm (Figure 7) with a coefficient of determination (R2) of 0.998. Higher concentrations were performed (from 20 ppm to 50 ppm), and our study showed that the method was no longer in the linear range.
FIGURE 7: Plot showing method linearity (R2 = 0.9983).
The method sensitivity was evaluated through analyzing the LOD and the lowest level of quantitation (LOQ). The method LOD and LOQs are 0.5 and 1 ppm, respectively.
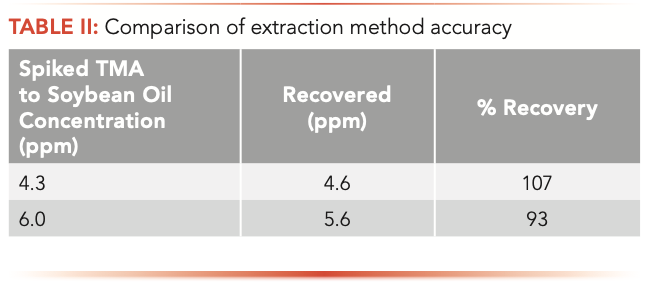
In the production of fish oil softgels, soybean oil has been used to dilute fish oil to achieve the desired EPA and DHA level in the finished product. Therefore, soybean oil was used as a fish oil product placebo. To assess the extraction efficiency of the proposed method and matrix effects, soybean oil was spiked with trimethylamine at 4.3 and 6 ppm concentrations. Satisfactory recoveries of ±7% were obtained for TMA (Table II).
Comparison of TMA Level in Fresh Fish Oil and Aged Fish Oil
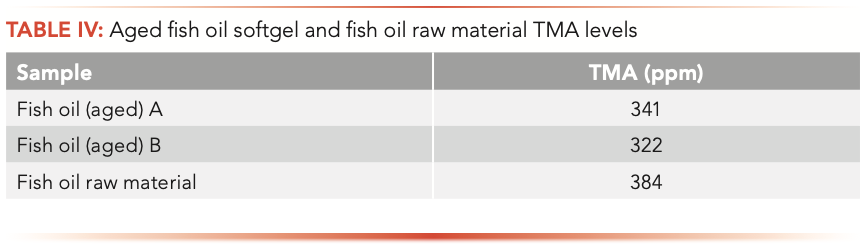
Aged samples and products stored at 40 oC/75 RH showed minimal change in their TMA levels. TMA levels in the finished products correlated with the amount of TMA in the fish oil raw material, which indicated that fish oil softgel production processes do not contribute to the TMA level. Choline in the product does not have an impact on TMA levels. TMA levels do not correlate with neither the age of fish oil and softgels nor the intense and rancid odor of the product (Table III and Table IV).
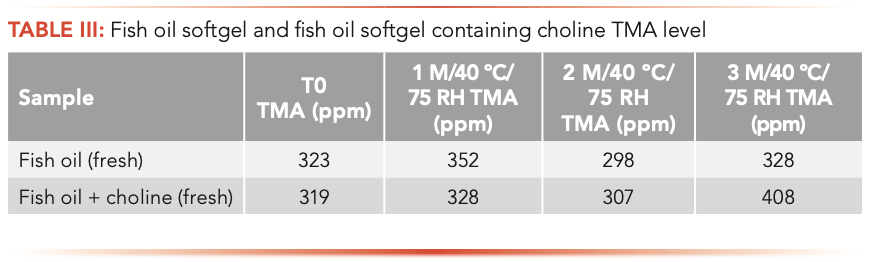
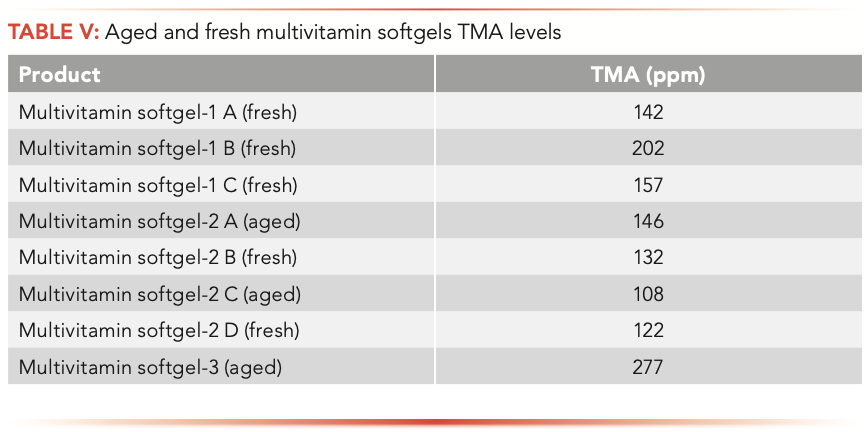
Fresh experimental multivitamin softgels containing fish oil and aged (24-month at room temperature) multivitamin softgels test results indicated that TMA levels in multivitamin softgels are lower than in fish oil products with a wide range of 108–277 ppm. The TMA level does not correlate with the product age and rancidity (Table V).
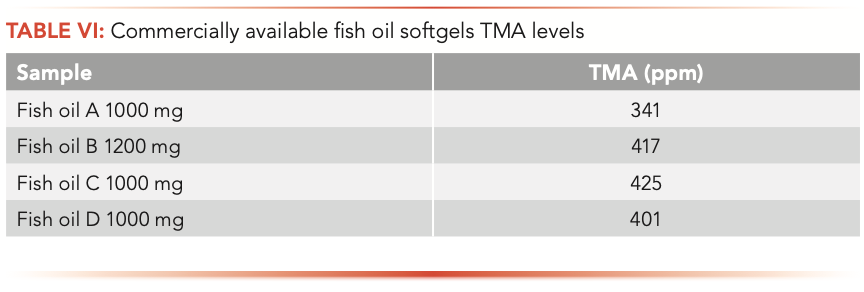
Further investigations were performed on various fish oil products that are available in the market. Table VI lists the TMA levels of four commercially available fish oil softgels ranging from 341 ppm to 425 ppm.
Conclusions
A reversed-phase HPLC method conjugated with derivatization was successfully developed to determine the TMA levels in fish oil softgels and multivitamin softgels containing fish oil. The method linearity range is 1–17 ppm with LOD and LOQ of 0.5 ppm and 1.0 ppm, respectively.
The levels of TMA in finished products do not change under accelerated and room temperature conditions. No difference between levels of TMA in the fish oil and fish oil with choline softgels were observed. Therefore, choline does not contribute to the level of TMA in the fish oil product containing choline. Fish oil raw material TMA level correlated with the TMA levels in fish oil finished products, which indicated the TMA levels do not increase during the fish oil softgel production.
Fish oil rancidity can be related to several factors such as aldehyde, secondary oxidant, and TMA. However, our research indicated that TMA levels do not correlate with the age or the level of rancid smell of the fish oil and multivitamin finished products.
Acknowledgment
The authors acknowledge Pharmavite LLC Analytical Services and their R&D department for their support to this research work.
Conflict of Interest Statement
This research was funded by Pharmavite LLC. All authors do not have financial conflicts of interest.
References
(1) D. Cameron-Smith, B.B. Albert, and W.S. Cutfield, J. Nutr. Sci. 4, e36 (2015).
(2) S.A. Beatty, J. Fish Res. Bd. Can. 4a, 63–68 (1938).
(3) D. Fennema, I.R. Phillips, and E.A. Shephard, Drug Metabolism and Dis- position 44(11), 1839–1850 (2016).
(4) A.Q. Zhang, S.C. Mitchell, and R.L. Smith, Dietary Food Chem Toxicol. 37, 515–520 (1999).
(5) R.J. Mackay, C.J. McEntyre, C. Henderson, M. Lever, and P.M. George, Clin. Biochem. Rev. 32(1), 33–43 (2011).
(6) W.J. Dryer, J. Fish. Res. Bd. Canada 6, 351–358 (1945).
(7) P Malle and S.H. Tao, J. Food Prot. 50(9), 756–760 (1987).
(8) W. Fiddler, R.C. Doerr, and R.A. Gates, J. Assoc. Off Anal. Chem. 4(2), 400–403 (1982).
(9) K. Oetjen and H. Karl, Deutsche Lebensmittel-Rundschau 95, 403–407 (1999).
(10) C. Chafer-Pericas, R. Herraez-Hernandez, and P. Campins-Falco, J. Chrom. A. 1023(1), 27–31 (2004).
(11) D.W. Johnson, J. Mass Spec. 43, 495–499 (2008).
(12) J. Yu, D. Wang, X. Gao, et al., J. Food Saf. and Qual. 8(2), 539–543 (2017).
(13) S. Veeravalli, K. Karu, I.R. Phillips, and E.A. Shephard, Science Direct 4, 310– 319 (2017).
Mina Fakhary, Fang Xia, and Mohamed Koroma are with Pharmavite LLC, in Valencia, California. Martin Dennison is with Pharmavite LLC in West Hills, California. Direct correspondence to: zfakhary@pharmavite.com

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).














