Pass the Salt: Evolution of Coupling Ion-Exchange Separations and Mass Spectrometry
LCGC Europe
Direct coupling of ion-exchange separations to mass spectrometric (MS) detection is increasingly being used for analyses of molecules ranging from organic acids to proteins. These approaches leverage both the exquisite selectivity of the ion-exchange mode for charge-based separation, and the tremendous power of mass spectrometry for identification of unknowns and trace-level quantitation.
Direct coupling of ion-exchange separations to mass spectrometric (MS) detection is increasingly being used for analyses of molecules ranging from organic acids to proteins. These approaches leverage both the exquisite selectivity of the ion-exchange mode for charge-based separation, and the tremendous power of mass spectrometry for identification of unknowns and trace-level quantitation.
The 48th annual International Symposium on High-Performance Liquid Phase Separations and Related Techniques, more commonly known simply as “The HPLC meeting”, was held in Milan, Italy, a few weeks ago (16–20 June). Upon preparing for the conference I thought about listening to the presentations this year with an ear towards ideas that could be helpful for people developing and troubleshooting liquid chromatography (LC) methods. As usual, I walked away from the conference with many research ideas, but also ideas for two topics I’d like to address in this column, this month and in September. The first topic is focused on the evolution of the coupling of ion-exchange separations with mass spectrometric (MS) detection.
During the HPLC meeting, there was an entire session dedicated to this specific topic, with six oral presentations covering aspects ranging from stationary phase development to development of MSâcompatible ion-exchange separations for molecules ranging from metabolites to intact monoclonal antibodies (mAbs). Clearly, there is a lot of interest in this area at the moment, and given that coupling ion-exchange separations to MS represents a significant change from the way ion-exchange has been practiced historically, we all have a lot to learn as we move forwards.
Introduction
Historically, the coupling of ion-exchange separations and MS has not been so straightforward because the high levels of nonvolatile salts that are typically used with many ion-exchange methods simply cannot be used with mass spectrometers. However, for some applications there is tremendous incentive to couple ion-exchange and MS because of the incredibly rich qualitative information MS detection can provide. Whereas the selectivity of ion-exchange separations can be very powerful (for example, one can easily separate 150 kDa mAbs that differ only by a single charged amino acid), if analytes are detected by a nonspecific detector (most commonly, UV absorbance), one is frequently left wondering, ”What is that peak?” Currently, this type of question is most commonly answered by collecting fractions of what is eluted from the ion-exchange column and re-analyzing them using a more MS-friendly separation technique such as reversed-phase LC. This process is very tedious and timeâconsuming, of course, and so the idea of directly coupling the ion-exchange separation to MS detection is attractive.
One might reasonably ask why nonvolatile salts are used so often when they are known to be incompatible with MS detection. The short answer to the question is that ion-exchange separations were developed long before electrospray MS was developed. There are still good chromatographic reasons to use particular nonvolatile salts over volatile ones in certain situations. However, in the early days of ion-exchange separations mobile phase additives were chosen on the basis of attributes other than volatility, such as transparency to optical detectors. For example, two of the most commonly used mobile phase additives for ion-exchange separations-sodium phosphate and sodium chloride-are highly transparent to UV light, and thus low wavelengths around 200 nm can be used to increase detection sensitivity when these additives are used. Furthermore, one of the other attractive attributes of phosphate salts is that phosphoric acid is a triprotic acid with pKa values at about 2.1, 7.2, and 12.3. The multiple pKa values and their spread across a wide pH range means that this single mobile phase additive can be used to effectively buffer the mobile phase pH across large pH ranges. Although phosphate cannot be used to provide buffering capacity over the entire range, it is frequently complemented with other (nonvolatile) additives such as citrate, which have pKa values that fill the gap between 2.1 and 7.2 for phosphate. Cocktails of such complementary additives can be prepared for use as soâcalled “universal buffers”. Unfortunately, there are no obvious alternatives to additives like phosphate and citrate that are both volatile enough to be used in high concentration with MS, and provide high buffer capacity across a wide pH range. Nevertheless, the attractiveness of coupling ion-exchange and MS directly for some applications has prompted several groups to investigate conditions that are viable for such separations. In the following sections, I provide examples of a few different lines of research in this direction.
Direct Coupling of IonâExchange and MS Using Mobile Phase Salt Gradients
The development of suppressor technology for ion-exchange separations (1) has had a tremendous impact on several application areas. Whereas much of the early research on this technology was focused on the analysis of inorganic ions using conductivity detection, more recent research has enabled high performance separations of organic ions coupled with MS detection. In the case of coupling ion-exchange with MS, the suppressor is important because it enables inline removal of nonvolatile inorganic components of the mobile phase (for example, potassium ions) prior to MS detection. This type of separation is increasingly being used in the metabolomics field for quantification of anionic metabolites, including nucleotides and sugar phosphates, where the results of ion-exchange MS separations complement those from other methods, including reversedâphase and hydrophilic interaction liquid chromatography (HILIC) separations coupled with MS detection (2,3). At the HPLC meeting in Milan, the last presentation in the session on coupling ion-exchange and MS by Hvinden described the use of cation-exchange separations for metabolomics (4).
Direct Coupling of Ion-Exchange and MS Using Mobile Phase pH Gradients and Volatile Buffers
Currently, the most active area of research on the coupling of ionâexchange and MS is focused on separations of biologics such as mAbs. Indeed, four of the six presentations in the session on coupling ion-exchange and MS at the HPLC meeting in Milan were focused on this topic in particular. Whereas small organic ions can be eluted from ion-exchange stationary phases with modest mobile phase concentrations of counterion (for example, 100-mM hydroxide in reference 3), larger molecules, such as proteins, can be more difficult to elute at a fixed pH if they are multiply charged in solution. For example, in our own work we have found that mAbs can be eluted from conventional cation-exchange phases using volatile buffers such as ammonium acetate (pH 6) that would be suitable for coupling to MS, but at rather high concentrations on the order of 0.2 M (5). While such mobile phases may be used with electrospray MS for a short time, prolonged use under these conditions is not recommended.
The high level of salt required for elution of proteins has motivated a number of groups to explore the potential to elute proteins from ion-exchange phases using gradients in mobile phase pH, rather than mobile phase salt concentration. The central principles that control retention and elution in this case are pretty straightforward. Consider the behaviour of a protein as an example. One of the important characteristics of a protein is its isoelectric point (pI)-the pH at which the net charge on the molecule in solution is zero. This does not mean it is uncharged; rather, it means that at the pI, the number of negative charges on the protein (from deprotonated carboxylate groups, for example) is equal to the number of positive charges on the protein (from protonated amino groups, for example). At pH levels well below the pI the protein will carry a net positive charge and be retained by a stationary phase designed for cationâexchange separations, and at pH levels well above the pI the same protein will be unretained. Moreover, changes to the protein that alter the pI, such as the addition or deletion of amino acids with ionizable side groups, or a change to an amino acid such as deamidation, will in turn influence retention in an ionâexchange separation. So, the strategy to separate two molecules having different pI values using a pH gradient and a cationâexchange stationary phase would be to first inject the sample into the column running with a mobile phase buffered well below the pI of the analytes, with relatively low salt concentration, so that they are retained. This could be something like 25-mM ammonium acetate, with the pH adjusted to 5 with acetic acid. Then, using a second buffer, such as ammonium carbonate adjusted to pH 10, a gradient of the two buffers is carried out such that the pH of the mobile phase mixture inside the column slowly increases from 5 to 10 over the course of the separation. The protein with the lowest pI should elute first, and the protein with the highest pI should be eluted last.
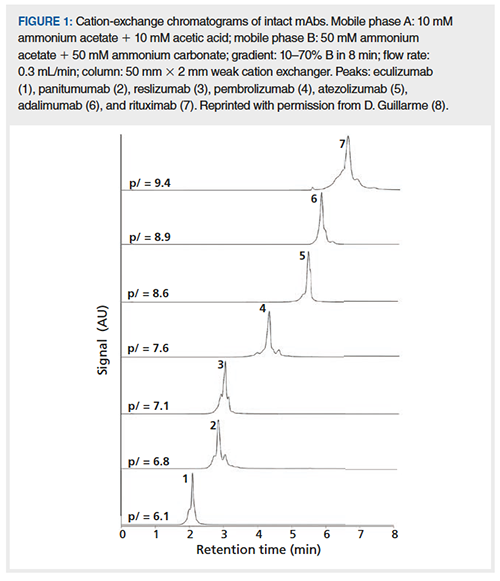
While the main principle of separating ionizable analytes using a pH gradient is straightforward, actually executing this in practice, with buffers that are MS-compatible, is where the difficulty lies. As discussed above, one of the virtues of phosphate as a buffering mobile phase additive is that phosphoric acid is a triprotic acid. This enables effective buffering of the mobile phase over much of the pH range that is useful for separations of biomolecules. On the other hand, MS-friendly additives, such as ammonium ion and formic acid, are only monoprotic acids, with pKa values that are far apart, and this limits their effectiveness when used in a pH gradient scenario. In spite of these challenges, in very recent work several groups have demonstrated the potential for direct coupling of cation-exchange separations to MS detection using volatile mobile phase additives and pH gradients (6–8). Much of this work to date has been focused on separations of mAbs. Farsang, Fekete, and coworkers compared the performance of mAb separations by cation-exchange with pH gradients using mobile phases prepared with different types of MSâcompatible additives (8). They report that the following single set of buffers enable separations of mAbs that span a range of pIs, with good retention, peak shape, and selectivity. Buffer A: 10 mM ammonium acetate + 10 mM acetic acid (unadjusted pH ~4.7); Buffer B: 50 mM ammonium acetate, 50 mM ammonium carbonate (unadjusted pH ~9.0). Note that a gradient made with these buffers will increase in both pH and salt concentration over the course of the gradient. Figure 1 shows representative chromatograms from their work, where the retention of the mAbs is highly correlated with pI, as expected. The ability to couple these chromatographic conditions-as a generic starting point in method development-with the identification power of mass spectrometry is indeed very powerful.
Indirect Coupling of Ion-Exchange and MS Using 2D-LC
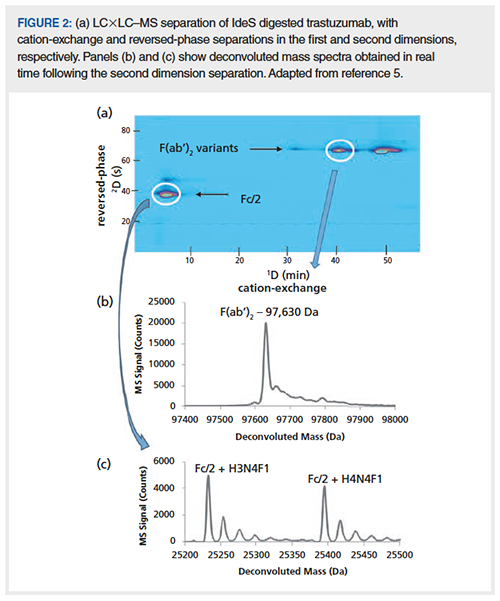
In the preceding two sections, I described recent research aimed at making the ion-exchange mobile phase itself more compatible with MS detection by replacing the commonly used nonvolatile mobile phase additives with volatile ones. A very different approach to obtain MS information about analytes separated by ion-exchange in real time is to use online two-dimensional chromatography (2D-LC). The virtue of this approach is that existing ion-exchange methods (or others, such as size-exclusion [SEC]) can be used as they are, where high mobile phase concentrations of nonvolatile additives may be required to improve peak shape (9). Briefly, in online 2DâLC, fractions of effluent from the first dimension column are collected in loops or traps and transferred (injected) into a second dimension column for further separation. In a simple case the second dimension separation can be a short, fast desalting step using reversed-phase or SEC conditions, for example, where the role of the second separation is to simply separate nonvolatile components of the first dimension effluent from the analytes of interest. The second separation can also be much more powerful, of course, and provide additional separation of analytes that were not resolved by the first separation. An example of such a separation from our own work is shown in Figure 2, where a salt gradient of ammonium acetate at pH 6 was used to elute mAbs from a first dimension cation-exchange column, followed by a second dimension reversed-phase separation running with 0.1% formic acid as a mobile phase additive, prior to MS detection (5). In this case the second dimension separation not only separates the high concentration of ammonium acetate from the protein analytes of interest, but also separates subunit variants of the mAb that were not separated by cation-exchange. Readers interested in learning more about 2D-LC are referred to recent review articles that describe the state of the art (10,11).
Closing Thoughts
Each year I attend the HPLC meeting, I walk away with a lot of excitement about the innovative and powerful things researchers are doing with liquid chromatography, which is sometimes perceived as a mature technique. In fact, there are many exciting developments each year, and the evolution of coupling ion-exchange separations directly to MS detection is one development that should be interesting to follow in the years to come.
References
- H. Small, T.S. Stevens, and W.C. Bauman, Anal. Chem.47, 1801–1809 (1975). doi:10.1021/ac60361a017.
- H.F.N. Kvitvang, K.A. Kristiansen, and P. Bruheim, J. Chromatogr. A 1370, 70–79 (2014). doi:10.1016/j.chroma.2014.10.029.
- C. Petucci, A. Zelenin, J.A. Culver, M. Gabriel, K. Kirkbride, T.T. Christison, and S.J. Gardell, Anal. Chem.88, 11799–11803 (2016). doi:10.1021/acs.analchem.6b03435.
- I.C. Hvinden, J. Walsby-Tickle, S. Liu, C.J. Schofield, and J.S.O. McCullagh, Developing Cation Exchange Chromatography Coupled to Mass Spectrometry for Metabolomics Applications, (2019). https://www.hplc2019-milan.org/ (accessed 10 July 2019).
- M. Sorensen, D.C. Harmes, D.R. Stoll, G.O. Staples, S. Fekete, D. Guillarme, and A. Beck, MAbs8, 1224–1234 (2016). doi:10.1080/19420862.2016.1203497.
- Y. Yan, A.P. Liu, S. Wang, T.J. Daly, and N. Li, Anal. Chem.90, 13013–13020 (2018). doi:10.1021/acs.analchem.8b03773.
- F. Füssl, K. Cook, K. Scheffler, A. Farrell, S. Mittermayr, and J. Bones, Anal. Chem.90, 4669–4676 (2018). doi:10.1021/acs.analchem.7b05241.
- E. Farsang, A. Mursier, K. Horvath, O. Colas, A. Beck, D. Guillarme, and S. Fekete, LCGC Europe32(s5), 29–34 (2019).
- P. Petersson, K. Haselmann, and S. Buckenmaier, J. Chromatogr. A1468, 95–101 (2016). doi:10.1016/j.chroma.2016.09.023.
- D.R. Stoll and P.W. Carr, Anal. Chem.89, 519–531 (2017). doi:10.1021/acs.analchem.6b03506.
- B.W.J. Pirok, D. Stoll R., and P.J. Schoenmakers, Anal. Chem.91, 240–263 (2019). doi:10.1021/acs.analchem.8b04841.
Dwight R. Stoll is the editor of “LC Troubleshooting”. Stoll is a professor and co-chair of chemistry at Gustavus Adolphus College in St. Peter, Minnesota, USA. His primary research focus is on the development of 2D-LC for both targeted and untargeted analyses. He has authored or coauthored more than 50 peer-reviewed publications and three book chapters in separation science and more than 100 conference presentations. He is also a member of LCGC’s editorial advisory board. Direct correspondence to: LCGCedit@mmhgroup.com

Troubleshooting Everywhere! An Assortment of Topics from Pittcon 2025
April 5th 2025In this installment of “LC Troubleshooting,” Dwight Stoll touches on highlights from Pittcon 2025 talks, as well as troubleshooting advice distilled from a lifetime of work in separation science by LCGC Award winner Christopher Pohl.
Mobile Phase Buffers in Liquid Chromatography: A Review of Essential Ideas
December 11th 2024In this installment of "LC Troubleshooting," Dwight Stoll discusses several essential principles related to when and why buffers are important, as well as practical factors, such as commonly used buffering agents, that are recommended for use with different types of detectors.
In-Loop Analyte Degradation in Two-Dimensional Liquid Chromatography: Example and Solutions
September 13th 2024In this installment of “LC Troubleshooting,” we describe an artifact that can arise because of compound degradation during the transfer of fractions of the first dimension (1D) column effluent to the 2D separation.





