Online Ion-Exchange Solid-Phase Extraction Cleanup for PFAS in Food of Animal Origin
Commission regulation (EU) 2020/2388 amends Regulation (EC) 1881/2006 lowering maximum levels for a selection of perfluoroalkyl substances in food of animal origin to 0.2 µg/kg. This article describes automated solid-phase extraction (SPE) based on a weak anion-exchange sorbent and performed as an additional clean-up step and combined with liquid chromatography tandem mass spectrometry (LC–MS/MS). Cartridge wash with organic solvents reduced matrix effects considerably and quantification limits of 0.01–0.05 µg/kg were reached. Validation for meat, egg, and fish was performed in accordance with the EURL guidance document for per- and polyfluoroalkyl substances (PFAS) in food and feed.
The special physicochemical properties of per- and polyfluoroalkyl substances (PFAS) make them well suited for many household and industrial applications, as well as for a wide range of consumer products. PFAS have enjoyed widespread use, and their unique chemical properties make them difficult to break down. PFAS are ubiquitous. The lack of environmental degradation in combination with high solubility in water has resulted in a global distribution. PFAS are found not only in the environment but also in food and animal feed, in humans, and in wildlife. PFAS are toxic, and acute or long-term exposure could have detrimental health effects. Authorities worldwide are regulating their use and emissions into the environment (1,2). In addition, food and drinking water must be monitored for their presence.
An earlier publication in this magazine (3) demonstrated the potential of online solid-phase extraction (SPE) for the determination of PFAS in water in the context of the EU Drinking Water Directive (EU 2020/2184). Online SPE relies on small cartridges that can be eluted directly onto the high performance liquid chromatography (HPLC) column, enabling quantitative transfer of analytes to the analysis system. Extraction and enrichment of PFAS analytes from only 1 mL water allows quantification limits below 1 ng/L for all compounds listed in the directive (4). SPE improves the analysis not only based on the concentration factor but also by eliminating accompanying matrix compounds from the extract. The clean-up effect increases if the sorbent in the SPE cartridge differs from the one used in the analytical column. By using weak anion-exchange (WAX) resins, the SPE cartridges can be very efficiently washed with organic solvent after loading the sample.
This work investigates the possibility of reaching the low PFAS levels required by the EU commission regulation 2020/2388 (5) using online SPE–liquid chromatography–mass spectrometry (LC–MS) instead of direct injection when analyzing food extracts of animal origin. The fully automated sample preparation method used did not add to the overall analysis time once the first sample was injected into the HPLC system.
Experimental
The automated system consisted of a MultiPurpose Sampler (MPS Robotic Pro, Gerstel) and an online SPE system (SPExos, Gerstel) coupled to an LC–MS/MS system (1290 Infinity II Pump and LCMS 6495C, Agilent Technologies). SPE elution was performed using 0.25% ammonia in methanol delivered from a separate isocratic HPLC pump (Infinity II 1260 Iso Pump, Agilent Technologies). The eluate was merged with the starting level buffer from the binary analytical pump in a valve fitted with a T-rotor located inside the SPExos system. The analytical column used for the online SPE–LC–MS/MS process was a 3 × 100 mm, 2.7-µm Poroshell 120 EC-C18 (Agilent Technologies). For direct injection, a 2.1 × 50 mm, 1.8-µm Zorbax Eclipse Plus C18 column (Agilent Technologies) was used. Between the binary pump and MPS, a 4.6 × 50 mm, 2.7-µm Poroshell 120 EC-C18 delay column (Agilent Technologies) was installed. For the extract cleanup, Gerstel SPExos online SPE cartridges (Polymer Wax, Gerstel P/N 018804-023-00) were used.
All standards (Table 1) were purchased as solutions from Wellington Laboratories (distributed by Campro Scientific). Mixtures of isotopically labelled PFAS were used as internal standards for extraction and injection. Food samples (egg, meat, and fish) were processed in the following way: A 5 g sample, fortified with internal standard for extraction, was extracted twice with acetonitrile under alkaline conditions. After phase separation with sodium chloride, the combined organic phase was acidified with formic acid and frozen overnight. A first cleanup with dispersive SPE using MgSO4 and graphitized non-porous carbon was followed by evaporative concentration to 0.3 mL, and, after addition of internal standard for injection, brought to a final volume of 1 mL. Calibration in the range of 0.025–5 ng/mL (internal standards at 1 ng/mL) corresponds to 0.005–1 µg/kg in food.
The online SPE workflow consisted of initially conditioning the cartridge, first using 0.25% ammonia in methanol, then methanol, and finally water. After injection of the sample into the loop, it was loaded onto the cartridge using water, and the cartridge was then washed. These steps were all performed by the high-pressure dispenser (HPD) unit of the SPExos. Direct injection was performed using a second injection valve equipped with a 2 µL sample loop. One method with and one without cartridge wash were used to compare the extract clean-up efficiency. After the elution phase was completed, the chromatography was started and the binary pump delivered a gradient flow of 0.6 mL/min, employing water with 0.1% formic acid and methanol with 0.25% ammonia and 0.05% formic acid.
Detection was made in dynamic multiple reaction monitoring mode (dMRM). For each target compound and each isotope labelled internal standard, two MRM transitions were chosen, one quantifier and one qualifier (except perfluorobutanoic acid [PFBA] and perfluoropentanoic acid [PFPeA], for which only one transition had sufficient intensity).
Results and Discussion
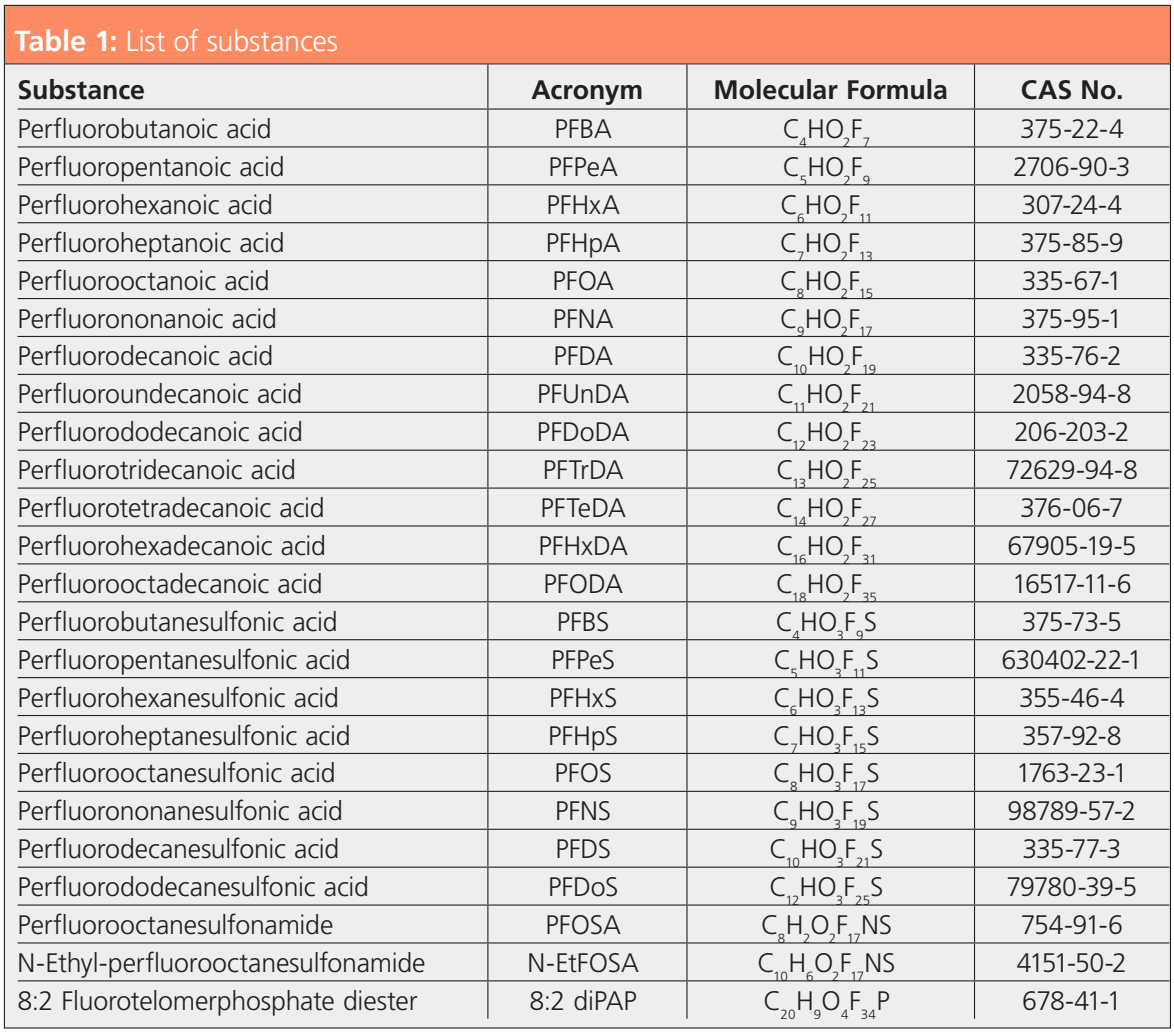
In online SPE, elution is usually performed using a gradient delivered by the analytical pump. The WAX cartridges used in this work were eluted with ammonia in methanol delivered by a separate (isocratic) HPLC pump. The eluate was subsequently merged with the mobile phase at the starting level buffer strength delivered by the binary analytical pump. The chromatography on the analytical column was based on a reversed phase separation, with molecular interactions that were very different from the ionic interactions with the WAX cartridge. This allowed larger sample amounts to be injected without encountering matrix effects. An injection volume of 25 µL was used compared to 2 µL for direct injection. As a result, not only were the signals increased by a factor of 10 to 13 compared to direct injection but signal-to-noise ratios were also improved on the same scale. The example in Figure 1 shows mass traces for perfluorooctanesulfonic acid (PFOS) in egg resulting from direct injection and online SPE injection of the same extract. As can be seen in the qualifier trace, interferences were reduced in intensity with a retention time shift.
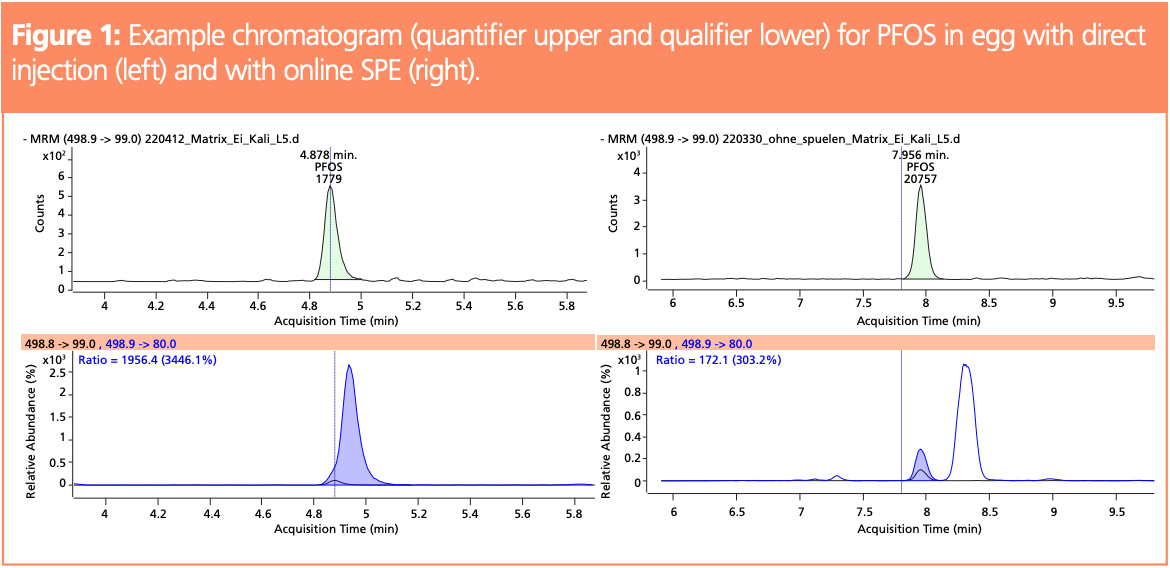
Acidic compounds were retained on the WAX cartridge by ionic interactions, which meant that the cartridge could be washed with organic solvents, resulting in the removal of most low and medium polar matrix components. Wash cycles with 300 µL of a mixture of 50:50:1 (v/v/v) acetonitrile–acetone–formic acid, 300 µL 0.01% formic acid in methanol, and 300 µL acetonitrile were used, respectively. Figure 2 shows total ion chromatograms with and without cartridge wash, demonstrating a considerable clean-up effect.
To investigate the matrix effects, method calibrations based on the individual sample types were performed in an identical manner and compared to method calibrations obtained from standards in solvent in the range of 0.05–1 ng/mL (corresponding to 0.01–0.2 µg/kg in food, based on a 5 g sample). All solutions were analyzed both by direct injection and online SPE, and both with and without cartridge wash. Some analytes showed considerably larger peak areas when cartridge wash was performed, indicating that matrix induced ion suppression was a factor.
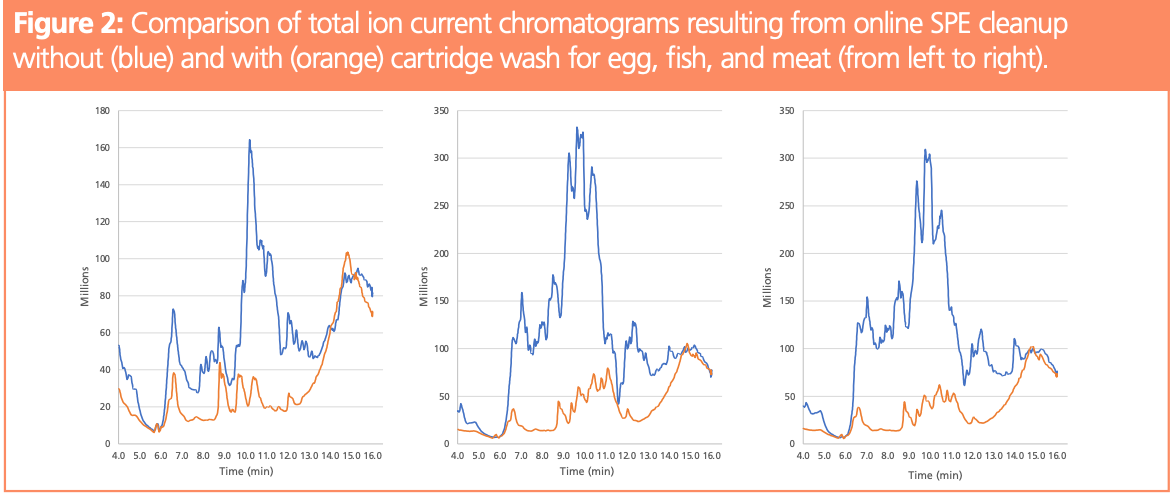
Matrix calibration is not always possible and most often a solvent-based calibration is used, including a correction based on internal standards. If an isotopically labelled analyte is available, the relative response can be determined independent of possible matrix effects. Whenever the internal standard is only similar to the analyte, the relative responses can differ, and proper quantification is not necessarily possible. An example is perfluorohexadecanoic acid (PFHxDA), which was quantified using 13C2-PFTeDA. The calibration curves obtained for the three matrices were all different and they all differed from the calibration curve based on solvent standards (Figure 3, left). Washing the cartridge before elution reduced matrix effects and, as a consequence, the slopes of the obtained calibration curves were similar (Figure 3, right).
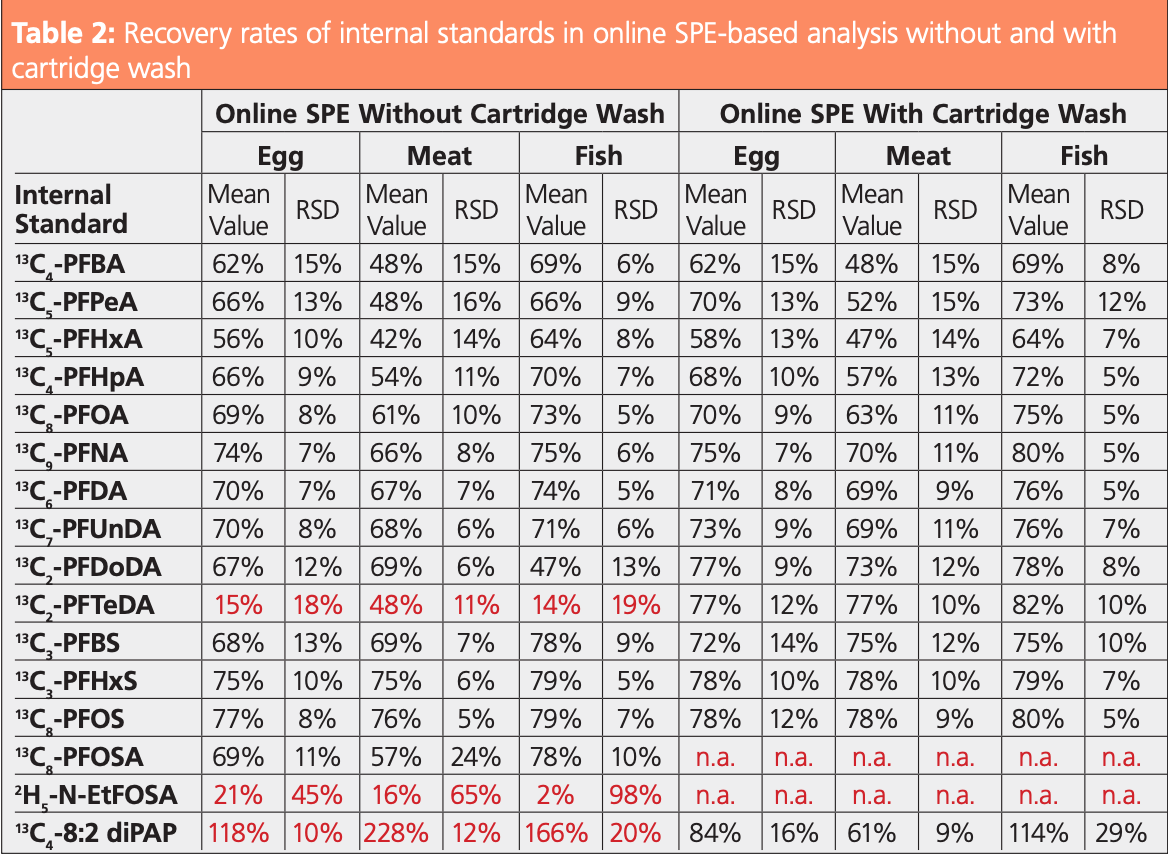
According to the EURL guidance documents (6), the limit of quantification (LOQ) represents the minimal concentration for which all quality criteria are met. Spiked samples at different concentration levels were analyzed in replicate to determine trueness and repeatability. Recovery of internal standards must be in the range 30–140%. As can be seen in Table 2, without cartridge wash the recovery of some compounds was very low (13C2-PFTeDA) or very high (13C4-8:2 diPAP) due to matrix effects. The cartridge wash improved the recovery rate of these internal standards considerably, resulting in reduced variations. (Non-ionic analytes were removed from the cartridge during the wash procedure and could therefore only be analyzed without using cartridge wash.)
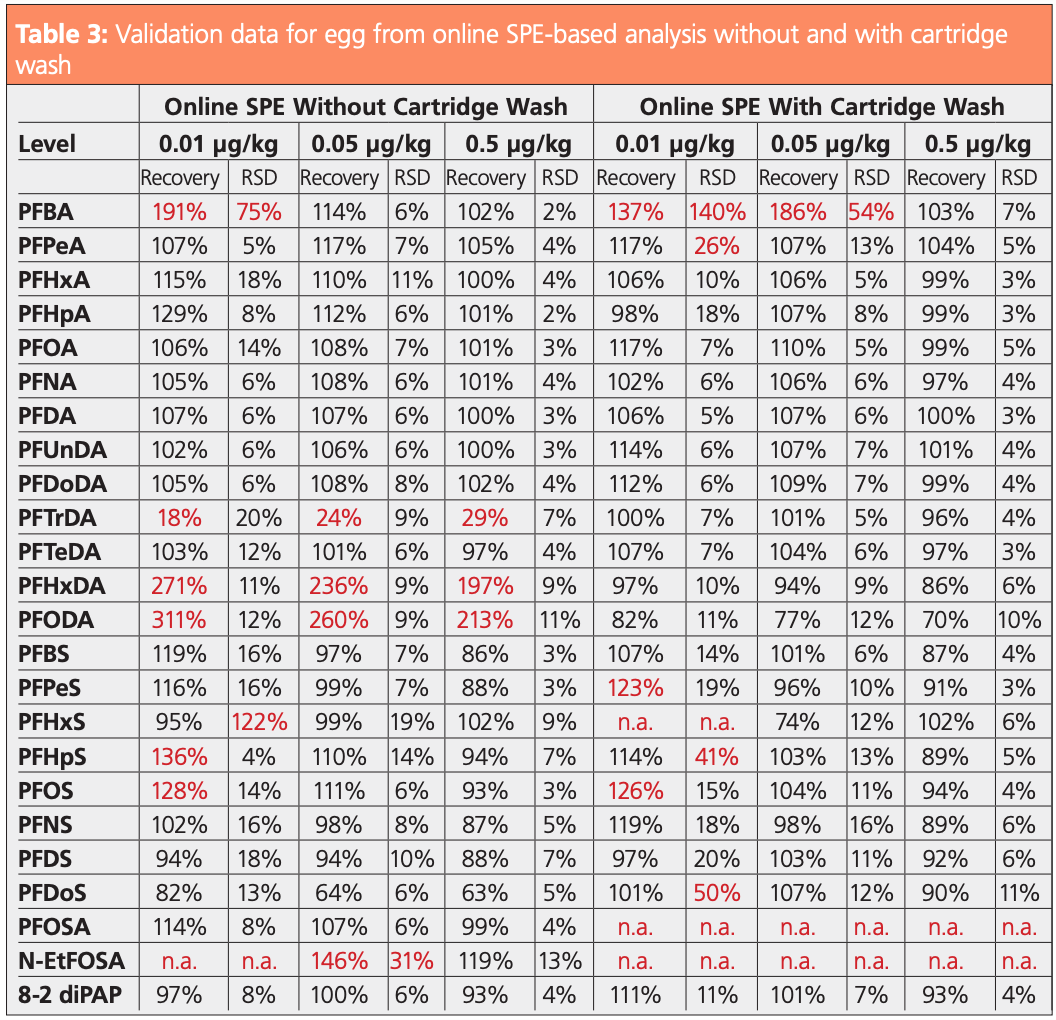
For a successful validation, repeatability and trueness must both be below 20%. As an example, validation results for egg using online SPE with and without cartridge wash are shown in Table 3. (More validation data can be found in reference 7.) As can be seen, for some compounds the validation was only possible when a cartridge wash was included. Most compounds were validated at levels as low as 0.01 µg/kg, with a few at 0.05 µg/kg. Due to blank values in the solvents used, for PFBA and PFPeA the LOQ was found to be lower without cartridge wash. N-Ethylperfluorooctanesulfonamide (N-EtFOSA) was validated at 0.5 µg/kg without cartridge wash.
Quantification limits were calculated for egg, meat, and fish based on determination with online SPE without and with cartridge wash. LOQs with cartridge wash were 0.01 µg/kg for most compounds, which was lower than the levels obtained without cartridge wash, and much lower than the levels reached by direct injection. Blank values introduced by the solvents used increased the LOQ for PFOA to 0.02 µg/kg and for PFPeA to 0.05 µg/kg. Without cartridge wash, the blank values for PFBA were lower, allowing a quantification limit of 0.05 µg/kg. LOQs of 0.5 µg/ kg were achieved for N-EtFOSA without cartridge wash, except when analyzing fish samples, in which N-EtFOSA was not quantified in the expected concentration range.
Conclusions
Online SPE–LC–MS/MS combined with the presented method enabled automated cleanup of food extracts and determination of PFAS compounds in the ng/kg range. Due to the clean-up effect of online SPE, the quantification limits compared to direct injection were significantly lower. The organic wash of the cartridges prior to elution effectively removed matrix interferences and improved the accuracy of the results. The method accuracy and trueness were demonstrated for different food types of animal origin (egg, meat, and fish). The main benefits compared to traditional SPE are simple sample handling, very low solvent consumption, and excellent reproducibility.
References
(1) ECHA: European Chemical Agency, Per- and polyfluoroalkyl substances (PFASs). https://echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas (accessed 2023-03-13).
(2) United States Environmental Protection Agency, Perand Polyfluoroalkyl Substances (PFAS). https://www.epa.gov/pfas (accessed 2023-03-13).
(3) Brandsch, T. Determination of PFAS in Water According to EU 2020/2184 and DIN 38407-42 Using Online SPE–LC–MS/MS. The Column 2022, 18 (3), 8–14.
(4) Brandsch T.; Lerch, O. Determination of PFAS in Water According to EU 2020/2184 and DIN 38407-42 Using Online SPE–LC–MS/MS. Gerstel AppNote 237 2022.
(5) Commission Regulation (EU) 2022/2388 of 7 December 2022 amending Regulation (EC) No 1881/2006 as regards maximum levels of perfluoroalkyl substances in certain foodstuffs. https://eur-lex.europa.eu/eli/reg/2022/2388/oj (accessed 2023-03-07).
(6) EURL for Halogenated POPs in Feed and Food, Guidance Document on Analytical Parameters for the Determination of Per- and Polyfluoroalkyl Substances (PFAS) in Food and Feed. Version 1.2, 11 May 2022. https://eurl-pops.eu/news/guidance-document-pfas/guidance-document-pfas (accessed 2023-03-07).
(7) Sauer, C.; Pleger, C.; Frenzel, T.; et al. Determination of PFAS in Food of Animal Origin Using Online SPE Cleanup and LC-MS/MS. Gerstel AppNote 247 2022.
About the Authors
Thomas Frenzel is head of Department 2.5 at the Landesuntersuchungsanstalt für das Gesundheits-und Veterinärwesen (LUA) in Saxony.
Claudia Sauer is employed as a certified food chemist in Department 2.5.
Christin Pleger is a food chemist and worked as part of her scientific thesis at LUA Saxony.
Thomas Simat holds the chair of Food Science and Consumer Goods at the Technical University Dresden.
Oliver Lerch is head of automated sample preparation in the Innovation and Technology Department at Gerstel.
Thomas Brandsch works as an application scientist at Gerstel in the field of automated sample preparation for LC–MS.
Email: thomas_brandsch@gerstel.de
Website: www.gerstel.com

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










