Novel GC–MS Software Programs for Analyzing Seized Drug Samples
The role of software to identify and distinguish seized drug samples.
There has been a proliferation of novel psychoactive substances (NPS) in drug seizures. These drugs are synthesized with minor modification to the chemical structure of existing drugs. Specifically, many fentanyl derivatives have shown up in drug seizures including isomeric variations that produce nearly identical mass spectra. These compounds are generally present at lower concentrations making identification difficult. Gas chromatography–mass spectrometry (GC–MS) is the workhorse in forensic chemistry for identification of controlled substances. Using two software programs—retention time locking (RTL), and deconvolution reporting software (DRS)—the authors developed an analysis capable of identifying and distinguishing isomeric forms. An example distinguishing para-methylacetylfentanyl and ortho-methylacetylfentanyl is described.
Forensic chemists have been encountering new materials in their drug casework (1,2). These novel psychoactive substances (NPS) are generally variations of a known controlled substance where the core structure is intact, but modifications have been made to various positions of the core structure. The core structure creates structural and positional isomers and analogous drugs that retain the pharmacological effects on the body, but may have different analytical properties in terms of retention time and mass spectral appearance (3).
A significant finding seen in drug seizures is fentanyl. Fentanyl is many times more powerful than other opiates such as morphine or heroin. It is added to illicit drugs due to the powerful effects that can be experienced from small doses. As an unintended consequence, many more people are dying from the use of drugs laced with fentanyl. In addition to fentanyl, numerous fentanyl derivatives have been identified in drug seizures, including isomeric variations that produce nearly identical mass spectra.
The rise in the number of fentanyl derivatives, coupled with the fact that they can be present in samples at low concentrations, has made data processing a more complex process for identification and confirmation.
Preliminary testing generally consists of non‑mass spectral testing such as colour tests, which can give an indication as to the identity. As seized drug mixtures have become more complex, these preliminary tests provide much less in terms of drug identity; especially for instances where the active component, that is fentanyl or fentanyl derivatives, is present in relatively small amounts.
NMS Labs and Process Description
NMS labs is a forensic chemistry and forensic toxicology laboratory that has performed forensic testing for over 50 years. Their testing procedures are designed to meet the strict requirements of civil and criminal justice professions. NMS uses state-of-the-art instrumentation to provide results backed by NMS lab’s accreditation and expertise. The forensic chemistry division is a series of drug testing laboratories throughout the United States involved with testing at the local, state-wide, and national level. Drug testing involves the recommended flow of testing recommended by SWGDRUG to identify seized drugs, starting with standard preliminary test procedures for initial identification. This includes various colour tests, pharmaceutical identification (where applicable), and, in certain cases, microscopic analysis. Confirmatory testing is generally performed by gas chromatography coupled to a mass spectrometer (GC–MS). The laboratories have additional testing capability such as liquid chromatography (LC)–MS/MS, Fourier-transform infrared spectrometry (FT-IR), and high performance liquid chromatography (HPLC)–UV. Additional identifying tests are run by the Center for Forensic Science Education and Research for identification of emerging drugs of interest. Testing is performed using LC–quadrupole time-of-flight (QTOF) mass spectrometry for more in-depth molecular identification.
GC–MS is the workhorse in forensic chemistry for the identification of controlled substances. This analysis platform has found favour due to three primary considerations: instrument cost, ease of use, and utility of multiple GC–MS databases from multiple sources. Electron ionization produces nearly identical mass spectra on multiple systems from multiple vendors. Most drug testing laboratories have adopted GC–MS for these reasons. Additionally, GC–MS is deemed a top tier assay for confirmation of drug identity. In the United States, the Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG) recommends that drug identification be performed using at least one test that provides structural information (designated a category A technique), which includes mass spectrometry (4).
As previously described, NMS Labs performs forensic chemistry testing and maintains a network of testing laboratories in the United States across multiple states. It is important that standardized analytical testing is provided at all sites. These sites analyze many thousands of samples annually, and it is necessary to have confidence that all samples are correctly analyzed and reported independently of where the sample was received. Standardized data acquisition and processing also allows inter‑laboratory technical review of casework.
The Analytical Approach to Testing
NMS Labs has established procedures for extraction and subsequent GC–MS analysis for seized drug evidence. The data processing procedure incorporates multiple software programs, retention time locking (RTL), deconvolution reporting software (DRS), and AMDIS (Automated Mass Spectral Deconvolution and Identification System) (5,6). Using these programs, the laboratory developed an analytical method capable of identifying and distinguishing many isomeric forms of evolving drugs.
RTL software allows for retention time reproducibility despite changes in column dimensions through variation in production and maintenance associated with normal use in the laboratory. Retention time locking calibration calculates how changes in pressure affect retention time. Using this calibration, slight alterations are made to the carrier gas flow by adjusting the pressure in the method and from there to the retention times of analytes. This can be used to accommodate column clipping and even installation of a new column. The RTL program will re‑calculate the pressure necessary to provide the desired retention time after each maintenance event. The laboratory can therefore maintain a database of drugs with the inclusion of retention time in the database entry. This method can subsequently be propagated to other instruments in the laboratory and to instruments throughout the network. As a result, when a case containing cocaine is analyzed at one facility in Pennsylvania, USA, the retention time of the analyte matches that of cocaine at all other facilities. As new compounds are detected and primary reference standards become available, the standard can be purchased, run on one system, added to the in‑house database, and propagated to all the laboratories with the confidence that the retention times will be the same on all the systems. This also makes it easy to review and, if necessary, re‑process data to verify findings.
Figure 1 illustrates the reproducibility of retention times with and without implementing RTL. The teal line shows the daily retention time noted for cocaine over a period of a month on three instruments using RTL. The dotted blue lines separate the data from one instrument to the next. The blue line shows the daily retention time noted for cocaine over a similar time period without the use of RTL. Note the changes in retention from instrument to instrument presented by the blue line. The teal line shows a constant retention time over the same period between instrumentation. Testing was performed on other compounds to demonstrate the same level of repeatability.

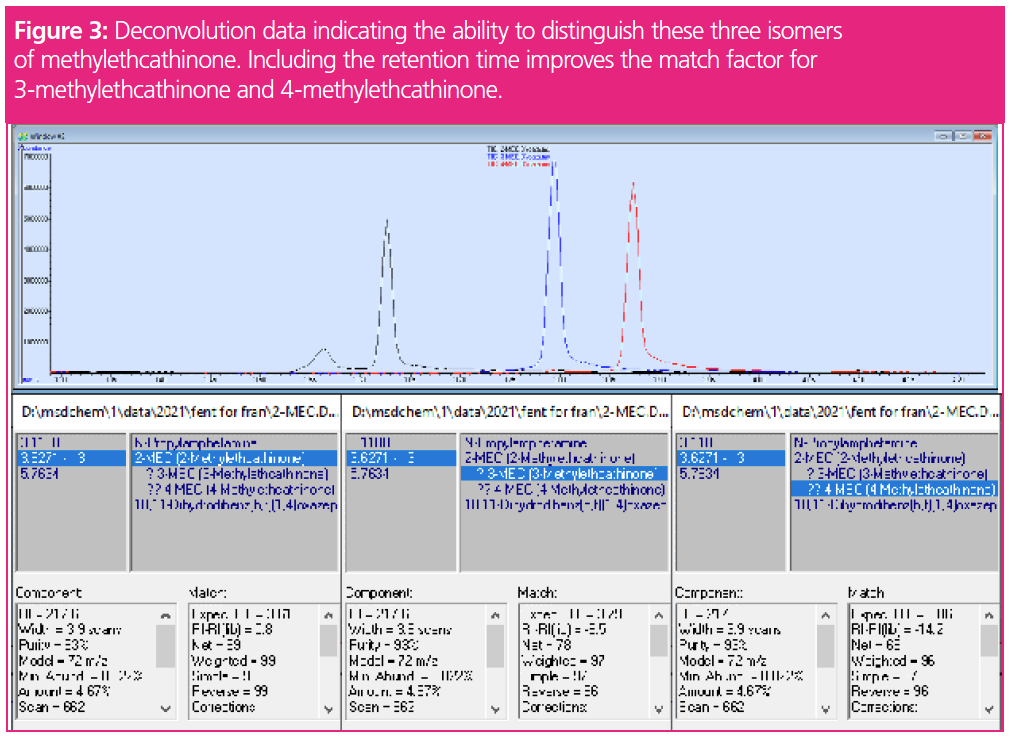
The second software application is the use of DRS and AMDIS. AMDIS provides a comprehensive means of searching data files for target compounds through an examination of extracted ion profiles to determine the presence of individual compounds. Deconvolution is achieved when masses exhibiting similar chromatographic profiles and retention times of the peak apex are grouped together as belonging to the same component (Figure 2). Masses with different peak shapes or retention times are not included in the deconvolved spectrum. This automatically removes background column bleed, and can help separate and distinguish two very closely or coeluting compounds and assign the associated masses for each of the components. In addition, the ability to include retention time in the match factor allows the software to distinguish between analytes that have similar mass spectra, but different retention times (Figure 3). The images in Figure 3 show the differences in retention time of these three isomers. The use of DRS provides a more objective approach to identification, minimizing subjectivity on behalf of the bench chemist. It also increases efficiency as deconvolution performs many of the processes typically manually executed by the forensic chemists, such as spectral subtraction.
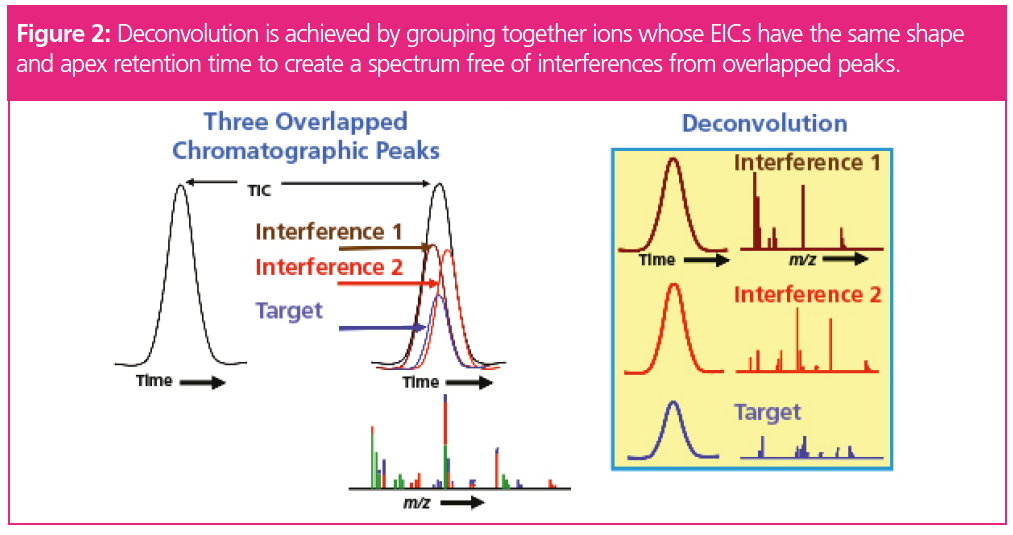
In addition to the increase in efficiency and objectivity, benefits for the laboratory network include the ability to share mass spectral databases where the retention times are essentially the same at each of the remote sites and on each individual GC–MS system. This shared database alleviates the need to acquire, run, and measure retention times of suspected controlled substances to determine the specific isomeric form. This, in turn, provides better turnaround time in reporting of results and saves individual laboratories the cost of obtaining standard reference material. As such, master databases can be built and managed at the corporate home laboratory and periodically updated and distributed to remote sites.
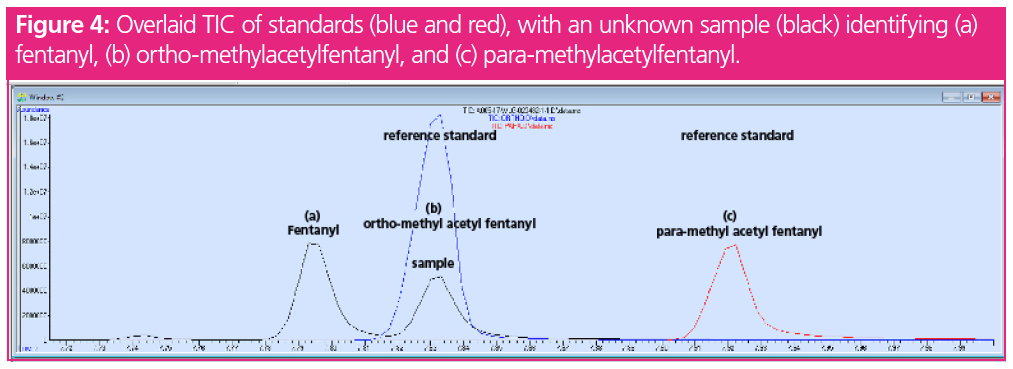
An example of how RTL assisted in distinguishing para-methylacetylfentanyl and ortho-methylacetylfentanyl is described. A sample was submitted for identification and was tentatively identified as methylacetylfentanyl based on a mass spectral database search. The only certified reference material available at the time of purchase was para-methylacetylfentanyl. Upon analysis, the unknown sample was found to have a retention time of 7.833 min. The retention time of the acquired para‑methylacetylfentanyl standard eluted at 7.922 min, a 0.089‑min difference. While a difference of 0.089 min may not seem like a large shift, as the method is retention time locked, the change in retention time indicated a misidentification. When looking at para-methylacetylfentanyl, there is the ability for the compound to have positional isomeric species in the form of ortho- and meta-methylacetylfentanyl. A standard of ortho-methylacetylfentanyl was later purchased and analyzed with the RTL locked method. The new standard eluted at exactly 7.833 min, the identical retention time of the unknown (Figure 4). Figure 4 is an overlay of the case sample, ortho-methylacetylfentany, and para‑methylacetylfentanyl. RTL was crucial in making this identification as the mass spectral data of these para- and ortho- isomers are identical, supporting the need for chromatography to aid in these identifications.
Conclusion
RTL and DRS coupled with AMDIS have been demonstrated to be valuable tools for analyzing complex seized drug materials. RTL ensures precise retention times over time and across multiple instruments. This improves the match factor, especially for chromatographically separated isomers, when combined with DRS, as shown for methylethcathinone. The combination of RTL and DRS also ensures accurate identification of unknown samples, as seen with the fentanyl derivates. Due to the ever‑increasing number of compounds found in seized drugs, this approach provides a more complete analysis for identification and detection.
For Forensic use.
References
- Drug Enforcement Administration, Emerging Threat Report, Third Quarter 2020 (Springfield, Virginia, USA, 2020).
- European Monitoring Centre for Drugs and Drug Addiction, European Drug Report: Trends and Developments (Lisbon, Portugal, 2020).
- M.G. Schmid and J.S. Hägele, J. Chromatogr. A. 1624, 461256 (2020).
- Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG), SWGDRUG Recommendations Version 8.0, (USA, 2019). https://www.swgdrug.org/
- “Retention Time Locking for GC Systems”, Agilent (Santa Clara, California, USA). https://www.agilent.com/en/retention-time-locking (Accessed 10 March 2021).
- “Deconvolution Reporting Software (DRS)”, Agilent (Santa Clara, California, USA). https://www.agilent.com/en/products/software-informatics/massspec-workstations/deconvolution-reporting-software-(drs) (Accessed 10 March 2021).
Francis Diamond is a senior staff scientist for the Center for Forensic Science Research & Education (CFSRE) in Pennsylvania, USA. Previously, Diamond was Technical Director in the Criminalistics chemistry division of NMS Labs, Pennsylvania, USA, working for NMS Labs for 43 years. His areas of expertise are in the techniques of separation and detection of drugs/controlled substances.
Abbey Fausett is an applications chemist for the Gas Phase Separations Division within Agilent Technologies. In her 10 years with the company, she has contributed to the company by taking roles in service, support, and marketing.

LCGC Blog: Forensics Laboratories Underassess Uncertainty in Blood Alcohol Determinations
August 8th 2023The level of uncertainty provided by most forensic laboratories for reported blood alcohol results has been woefully underassessed. Not only is this bad science, but someone’s civil liberties may be at stake.












