New Gas Chromatography Products for 2016
LCGC Asia Pacific
John Hinshaw presents his annual review of new developments in the field of gas chromatography (GC) seen at Pittcon and other venues in the past year.
John V. Hinshaw, GC Connections Editor
John Hinshaw presents his annual review of new developments in the field of gas chromatography (GC) seen at Pittcon and other venues in the past year.
The Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy (Pittcon) returned to Atlanta, Georgia, USA, for its 67th annual meeting on 6–10 March 2016. This year’s conference saw nearly 13,000 conferees and exhibitors in attendance. Interestingly, 37% of the participants categorized themselves as first-time attendees. Also of note were the 24% of attendees who hailed from foreign countries. The Pittcon exposition hosted 847 exhibitors from 37 countries in 1539 booths, including 119 first-time exhibitors. In addition, the Food Labs Conference was held in conjunction with Pittcon for the fourth year.
LCGC organized a half-day session devoted to the presentation of the 2016 LCGC Lifetime Achievement in Chromatography Award to Professor Milton L. Lee (Brigham Young University), and the LCGC Emerging Leader in Chromatography Award to Debby Mangelings (Vrije Universiteit Brussel). Detailed information on this year’s awards appears in the February 2016 issue of LCGC North America (1).
Following its well-established rotation of host cities, next year’s Pittcon will head north to Chicago’s McCormick Place, 5–9 March 2017, where participants will hopefully enjoy a spate of warm spring weather. Certainly, we will get that experience in 2018 when the conference goes down south to Orlando, Florida, for an early appearance from 25 February to 1 March.
This annual “GC Connections” instalment reviews gas chromatography (GC) instrumentation, columns, and accessories shown at this year’s Pittcon or introduced during the previous year. For a review of new products in other areas of chromatography, columns, and related accessories, please see the April 2016 issues of LCGC Europe and LCGC North America (2,3), which is also available on-line at www.chromatographyonline.com
The information presented here is based on manufacturers’ replies to questionnaires, as well as on additional information from manufacturers’ press releases, websites, and product literature about the past year’s products, and not upon actual use or experience of the author. Every effort has been made to collect accurate information, but because of the preliminary nature of some of the material LCGC Asia Pacific cannot be responsible for errors or omissions. This column instalment cannot be considered to be a complete record of all new GC products introduced this year at Pittcon or elsewhere because not all manufacturers chose to respond to the questionnaire, nor is all of the submitted information necessarily included here because of the limited available space and the editors’ judgment as to its suitability.
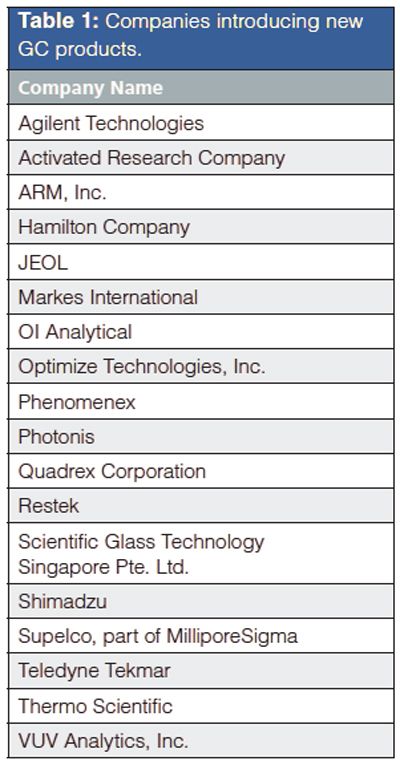
Gas Chromatography: 2015–2016
The number and type of new product introductions that were gathered for this review are an indication of the continuing viability of gas chromatography in the face of more recent major developments in small molecule analysis. GC is still the “go-to” standard for volatile and semivolatile compounds. Although new mini- or micro-GC systems were not in abundance in this year’s crop, introductions in selective detectors, including mass spectrometry (MS) detectors and otherwise, methodâspecific autosamplers and accessories, and highly selective column stationary phases certainly were.
Thermo Scientific, for example, introduced two major advances in their MS detector product line: the Q Exactive GC hybrid quadrupoleâOrbitrap GC–MS–MS system, and the TSQ 8000 Evo triple-quadrupole GC–MS system. Shimadzu also advanced their MS detector offerings with the GCMSâTQ8040 system with Smart multiple reaction monitoring (MRM). From Agilent Technologies, the 5977B GC–MSD system with the company’s High Efficiency Source extends the lower detection limit of the company’s single-quadrupole detector. The BenchTOF-Evolve system from Markes is a new time-of-flight mass spectrometer for GC or GC×GC. All five MS offerings represent significant advances in MS capabilities for the gas chromatographer in a variety of applications.
VUV Analytics released its new VUV PIONA+ application, which is built on the company’s vacuumâultraviolet (UV) detector, and provides groupâtype selectivity for the targeted paraffin, isoparaffin, olefin, naphthene, and aromatic hydrocarbon classes. OI Analytical and Teledyne Tekmar both introduced new purgeâand-trap concentrators that provide enhanced and advanced capabilities for this venerable application.
There were numerous new offerings in the GC detector realm. From Agilent, two chemiluminescent detectors, the 8355 sulphur chemiluminescence detector and 8255 nitrogen chemiluminescence detector, offer enhanced sensitivity, compatibility with Agilent’s latest laboratory GC systems, and simplified designs with fewer parts. OI Analytical came out with a secondâgeneration pulsed flame photometric detector (PFPD) that features a modular design and reduced gas-flow requirements. Shimadzu introduced its contactâfree ECD-2100 detector, which uses a sweep gas to minimize contact of the sample with the detector source and produces lower detection limits and longer maintenance intervals. Activated Research Company introduced its Polyarc catalytic postâcolumn reactor that converts all carbonâcontaining compounds exiting the column into methane, which simplifies many calibration problems as well as responding to carbon monoxide, carbon dioxide, and formic acid.
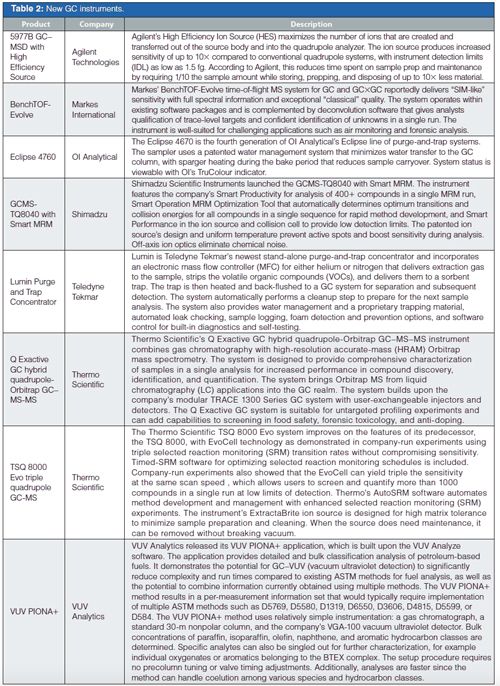
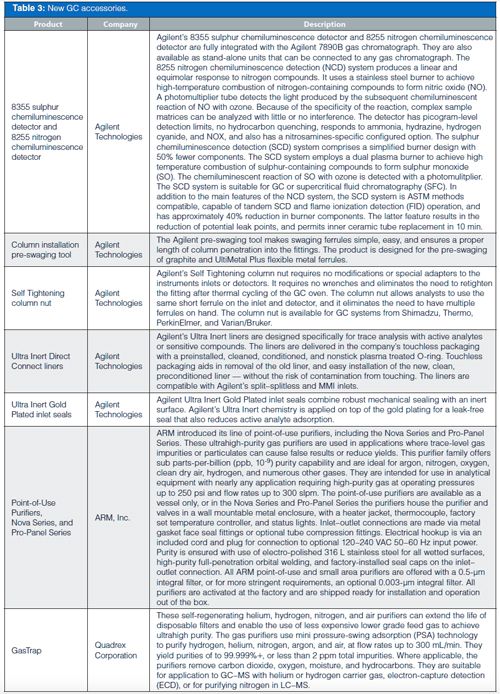
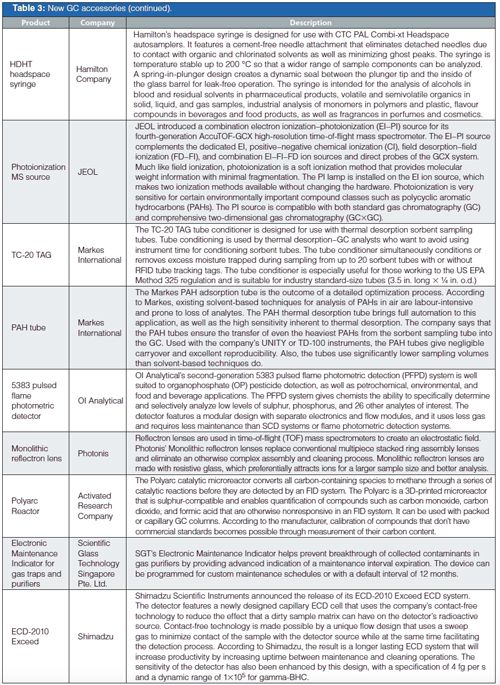
The GC accessories were rounded out by an interesting assortment of tools, inert liners, gas purifiers, thermal desorption tubes and conditioners, and syringes.
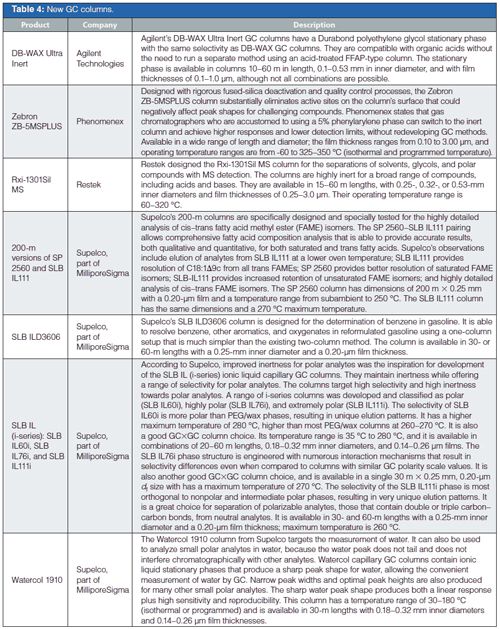
In GC columns, Supelco, part of MilliporeSigma, continues to expand its ionic-liquid (IL) column offerings with additional unique selectivities as well as a waterâspecific phase that is claimed to deliver a symmetrical water peak. Agilent, Phenomenex, and Restek all showed their latest columns with improved inertness and selectivity, plus in some cases higher temperature ranges.
All-in-all it was a healthy year for GC with the large number of varied offerings. I look forward to Pittcon 2017 and the opportunity to meet next years’s new class of GC products and technologies.
Acknowledgements
I would like to thank the manufacturers and distributors that kindly furnished the requested information, which allowed a timely report on new product introductions over the past year. For those manufacturers who did not receive a “New Products” questionnaire this year and would like to receive one and be considered for early inclusion into the 2017 new GC and related product introductions review, as well as the other related review articles to be published in LCGC, please send the name of the primary company contact, the mailing address, fax number, and e-mail address to Laura Bush, Editorial Director, LCGC and Spectroscopy, Advanstar Communications, 485 Rte. 1 South, Bldg. F, Suite 210, Iselin, NJ 08830, USA, Attn: 2017 New Chromatography Products. The questionnaire will be sent out in late 2016.
References
- M. L’Heureux, LCGC North Am.34(2), 128–143 (2016).
- D. Bell, LCGC Europe 29(4), 214–224 (2016).
- M.W. Dong, LCGC North Am.34(4), 262–273 (2016).
“GC Connections” editor John V. Hinshaw is a Senior Scientist at Serveron Corporation in Beaverton, Oregon, USA, and a member of LCGC Asia Pacific’s editorial advisory board. Direct correspondence about this column should be addressed to “GC Connections”, LCGC Asia Pacific, Hinderton Point, Lloyd Drive, Ellesmere Port, Cheshire, CH65 9HQ, UK, or e-mail the editor-inâchief, Alasdair Matheson, at amatheson@advanstar.com

Altering Capillary Gas Chromatography Systems Using Silicon Pneumatic Microvalves
May 5th 2025Many multi-column gas chromatography systems use two-position multi-port switching valves, which can suffer from delays in valve switching. Shimadzu researchers aimed to create a new sampling and switching module for these systems.
Troubleshooting Everywhere! An Assortment of Topics from Pittcon 2025
April 5th 2025In this installment of “LC Troubleshooting,” Dwight Stoll touches on highlights from Pittcon 2025 talks, as well as troubleshooting advice distilled from a lifetime of work in separation science by LCGC Award winner Christopher Pohl.
Study Examines Impact of Zwitterionic Liquid Structures on Volatile Carboxylic Acid Separation in GC
March 28th 2025Iowa State University researchers evaluated imidazolium-based ZILs with sulfonate and triflimide anions to understand the influence of ZILs’ chemical structures on polar analyte separation.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



