A Multimodal Liquid and Supercritical Fluid Chromatography Chiral Separation Screening and Column Maintenance Strategy Designed to Support Molecules in Pharmaceutical Development (Part 1)
LCGC Europe
Chiral separation screening has become a widely accepted approach for the rapid identification of an appropriate chiral stationary phase for use in more focused enantioseparation optimization. A set of extended screens encompassing various chromatographic modes using HPLC and SFC is presented.
Chiral separation screening has become a widely accepted approach for the rapid identification of an appropriate chiral stationary phase (CSP) for use in more focused enantioseparation optimization. A set of extended screens encompassing various chromatographic modes using high performance liquid chromatography (HPLC) and supercritical fluid chromatography (SFC) is presented. These multimodal screens are tailored to meet the specific and changing needs of customers as distributed along the drug development process within a typical pharmaceutical development setting. In addition, a systematic column testing and regeneration approach is presented and emphasized for maintaining the reliability of column and screen data. Comprehensive screening systems created to serve such an expansive customer base, actively supported with manufacturer-recommended column suitability and regeneration procedures, are not well represented in the literature.
Of the myriad analytical tests required to support a pharmaceutical candidate during the drug development process, the determination of a rugged and reliable enantioseparation method for a chiral compound is frequently the most challenging requirement. An arsenal of techniques is available for this task, including capillary electrophoresis (CE), supercritical and subcritical fluid chromatography (SFC), capillary electrochromatography (CEC), gas chromatography (GC), and high performance liquid chromatography (HPLC). Depending on specific factors such as compound structure, chemical and physical properties, and sample matrix, each respective technology possesses relative advantages and disadvantages pertaining to speed, efficiency, and sample compatibility.

Regardless of the technique used for enantiomer analysis, significant cost and expertise are inherent in the process. Herein is one distinct advantage among many for choosing HPLC. Since HPLC instrumentation is a mainstay of chromatography, one usually only needs to invest in the chiral columns to pursue an HPLC chiral separation. Admittedly, the columns can be costly, but not as expensive as the financial outlay that might be required to set up dedicated instrumentation using ancillary techniques. Another advantage of HPLC is its general familiarity among scientists. Most analytical chemists understand basic HPLC and can quickly use their familiar instrumentation towards the chiral separation. The proportion of scientists in a typical laboratory setting who are skilled in the competing techniques (mentioned earlier) is almost always notably less compared to those familiar with traditional HPLC.
An efficient approach to meeting the chiral separation demands imposed across the drug development arena should involve the various techniques aforementioned when they offer an advantage. However, the further a chiral molecule progresses along the drug development pipeline, the more the likelihood increases that traditional chiral HPLC will surface as the analysis technique of choice at compound registration. As the reliability of instrumentation supporting other separation techniques continues to improve, an increased presence of non-HPLC enantiomer analysis methodology will certainly permeate the downstream drug development process.
Presently, the superiority of chiral analysis via HPLC for most analytes continues to focus most of the development within this well-honed technique. As a result, a great number of HPLC chiral columns have been marketed in support of enantioseparation development. Even though certain chiral stationary phases (CSPs) are known to be more effective for selected compound classes, separation modes, or sample matrices, the search for the most suitable chiral selector for a given chiral analyte remains primarily a trial and error process. All is not blind, however. Ever-improving data repositories can provide direction in the search. Such chiral application databases can provide separation methods for compounds possessing structural similarity to those being studied by the chromatographer (1). Although these tools are certainly helpful for providing direction in the screening effort, the search for the ultimate enantioseparation remains time-consuming, inefficient, and expensive. An inadequate and noncomprehensive screening paradigm can result in a scientist unknowingly settling for an unnecessarily inferior separation system. The number of CSPs, when combined with compatible mobile phases, becomes exponential and moves beyond the scope of the typical time and resource-limited laboratory.
As a pragmatic response, the industry has generally approached HPLC chiral separation development with the use of various tailored efforts, usually built upon a brute screening approach using the more promising CSPs. Such screens are sometimes augmented by a flow scheme or decision tree offering specific guidance depending on the nature of the analyte (acid, base, neutral) or sample matrix (aqueous, nonaqueous). Ultimately, the experimental process is driven by the needs of the customer. A brief survey of published literature over the past decade reveals that the majority of HPLC enantioseparation screens are suited toward rapid development of methodology that can also be used at the preparative scale (2–12,14–16).
Within the realm of chiral preparative chromatography, analyte solubility and mobile phase volatility rise in importance to nearly the level of the quality of the enantioseparation itself. The use of polysaccharide-based chiral selectors with normal phase or polar-organic eluents frequently offers all of the above. Not surprisingly, many of the published chiral HPLC screens use columns of this class (5–10), a few of which are now off patent and available as "clones" with potential cost savings (11). While many in the industry utilize these CSPs in both normal-phase and polar-organic modes, the analogous polysaccharide coated columns designed for use in reversed-phase mode are occasionally substituted for polar-organic analysis (3,12,13). Some screens add a π-electron acceptor/π-electron donor CSP to their polysaccharide column screening repertoire (7,9,14). Literature reviews conducted by Akin and colleagues (17), Beesley (11), and Manglings and Vander Heyden (4) confirm the dominance of these CSPs, especially in the early stages of drug development. In the last few years, screens incorporating immobilized polysaccharide derivatives have been reported. Because of their immobilized nature, these columns allow for the use of a much broader range of solvents, notably enhancing capabilities, especially in polar-organic mode (8,10,15). The authors of this publication can report that more recently marketed immobilized CSPs also offer promise (18–20).
Chiral separation screening success using the predominately polysaccharide-style column set described above is unmatched, and remains a logical domain from which to operate, especially if preparative applications are in mind. Some chiral separation screens increasingly use SFC technology to take advantage of the technique's speed and "green" label earned because of its reduced solvent consumption (9–11). For laboratories primarily concerned with the quality of the enantiomeric separation at analytical scale, without preparative aspirations in mind, the choice of chiral selector and mode is greatly increased. The chromatographer's tool kit is greatly expanded once the reversed-phase mode is considered in tandem with compatible polysaccharide CSPs and other more specialized selectors indicative of this separation mode.
Samples dissolved in biological matrices, as well as more polar compounds with enhanced solubility in aqueous-based diluents, are excellent candidates for reversed-phase chiral methodology. Laboratories routinely tasked with chiral purity analysis are frequently already oriented towards standard achiral purity determination, much of which is conducted in the reversed-phase mode. In one aspect, the distomer (undesired enantiomer) could simply be considered as yet another impurity, albeit a special one. The advantage of using chiral analysis in reversed-phase mode, compatible with parallel achiral analysis in the same mode, is obvious. In such cases, instrument conversion to normal-phase solvents is avoided, as well as the tendency to "dedicate" an instrument to said chromatographic mode.
Broader Screening Driven by Customer Needs
The use of normal-phase, polar-organic, and SFC mode screening using primarily polysaccharide CSPs is logical if eventual preparative-scale enantioseparations are planned. Sound chiral methods optimized from these screening results are frequently utilized at analytical scale for chiral purity determination, either in support of concurrent asymmetric synthesis efforts or to measure fraction purity from preparative-scale chromatography batches. This tandem operation to supply quantities of chiral intermediates or test analytes is reasonably efficient and represents a logical approach in the early stages of the drug development pipeline. However, these analytical-scale chiral methods are frequently used much further into the development of a particular drug, frankly, when the needs of enantiomer analysis have changed in scope.
As a synthetic route is fine-tuned, and as a drug candidate nears regulatory filing, the needs of chiral methodology shift more and more towards tailored sample analysis and optimized distomer and impurity sensitivity (21). Methodology designed to support compounds later in development must be reliable, rugged, and transferable as dictated by the requirements of analytical quality by design. Many times, the chiral methods relied upon from the genesis of a particular drug are perhaps no longer suitable under such increased scrutiny. The ever-increasing regulatory demands placed on the pharmaceutical industry to develop the best in analytical methodology behoove the scientist at this venture to explore all chiral separation techniques available. Clearly, the well-developed, relatively rugged, and transferable nature (between laboratories) of HPLC-based enantioseparations is appealing. Often times, a counterpart to the discovery-based laboratory experienced in multi-faceted chiral separation development simply does not exist downstream. This is unfortunate, because the need for the identification of superior analytical-scale chiral separations exists precisely at this juncture.
Many times, the responsibility for identifying this second-generation separation system is left to the same analysts and laboratories also tasked with providing various other analytical techniques in support of the drug candidate and its synthetic intermediates. These are qualified scientists indeed, but not specialists possessing specific experience in the craft of analytical-scale chiral separations. What frequently results over time is the sprouting of multiple "silos of expertise" in which various laboratories of limited experience and resources explore the complex arena of chiral separations, suited solely to their specific and occasional needs. Such a process is highly inefficient, expensive, redundant, and sometimes only marginally successful.
A solution proposed by the authors and increasingly embraced within the pharmaceutical industry is the formation of a secondary chiral screening and optimization group positioned downstream of early discovery operations. Such a group, while it shares a great deal of the same technology used by the original discovery-based laboratory, additionally embraces a broader scope of chiral separation possibilities potentially amenable to nonpreparative enantioseparations. For instance, chiral separations in the reversed-phase mode, which are generally undesirable for preparative applications, can offer additional separation options in the analytical laboratory. In addition, because the reversed-phase chromatographic mode is heavily utilized in most analytical laboratories for achiral analyses, the presence of instrumentation readily available for reversed-phase chiral analysis is almost guaranteed.
Despite the increased separation options available to the analytically focused laboratory, the previously developed methodology supplied by the discovery laboratory can often provide valuable insight towards the downstream screening and optimization effort. In fact, because historically the better chiral separations are often obtained using polysaccharide CSPs in normal-phase or polar-organic mode (the bulwark of chiral purification laboratories), frequently the same basic separation developed in the original laboratory can be maintained or simply optimized for later use. This possibility should certainly be explored, but not summarily relied upon. One never knows if a more suitable chiral separation might be easily uncovered via expanded screening.
Experimental
Instrumentation: Various instrumentation formats are used, each platform dedicated to screening operations in a specific chromatographic mode. All normal-phase and some polar-organic and reversed-phase mode screening are conducted using an Agilent 1100 series HPLC system equipped with an autosampler and photodiode-array detector controlled by Empower software (Waters). Two external six-port Power Selector Elite column switchers (Analytical Sales & Services) allow for sequential screening using 12 different CSPs. Additional polar-organic and reversed-phase screening platforms use Agilent 1260 Infinity series HPLC systems with two 1290 Infinity four-port column switchers and Agilent Chemstation software.
The SFC instrumentation consists of an Agilent 1100 series HPLC system equipped with an autosampler and photodiode-array detector mated to a Fusion A5 SFC module controlled by Chemstation software. In addition, 12-column screening capability is provided by two Power Selector Elite six-port column switchers. The SFC and 1260 Infinity platforms are augmented with Agilent 12/13 12-port solvent selector valves, allowing for screening using a variety of eluent combinations.
Chiral Stationary Phases: The polysaccharide-based Chiralcel and Chiralpak CSPs were purchased from Chiral Technologies Inc. Lux Amylose-2, Cellulose-2, and Cellulose-4 polysaccharide columns were obtained from Phenomenex Inc. Regis Technologies Inc., provided the Whelk-O 1 brush-type CSP. The macrocyclic antibiotic Chirobiotic columns, Cyclobond I 2000 cyclodextrin-based CSPs, and the polymeric PCAP column were purchased from Supelco Analytical. All columns used in the screens are composed of 5-μm particle size material, measure 4.6 mm in diameter and 150 mm in length, except for the Whelk-O 1 column (250 mm).
Materials: HPLC-grade solvents (purity of 99% or greater unless indicated otherwise) are used for all chromatographic screens, separation suitability tests, and column regeneration regimens. Acetonitrile and methanol were obtained from EMD Millipore Corporation. Ethanol (200 proof) was purchased from Decon Labs, Inc. Isopropanol and n-hexane (95%) were purchased from Fisher Scientific, Inc. N,N-Dimethylformamide was acquired from Fluka Chemical Corporation. Ethyl acetate was purchased from Honeywell Burdick & Jackson Inc. Deionized water was supplied through a centralized laboratory purification system. USP-grade carbon dioxide was acquired from Airgas Mid America.
The basic eluent additives diethylamine were purchased from Sigma-Aldrich Corporation and triethylamine from EMD Millipore Corporation. The acidic eluent additive trifluoroacetic acid was acquired from Alfa Aesar. The chiral analytes used for HPLC column suitability tests were obtained from various suppliers. DL-Aminoglutethimide was purchased from Tokyo Chemical Industry Co. Ltd. DL-Laudanosine was acquired from Biosynth Chemistry and Biology International. 1,1'-Bi-2-naphthol and trans-stilbene oxide were acquired from Acros Organics. Benzoin, hydrobenzoin, flavanone, and 5-methyl-5-phenylhydantoin were purchased from Sigma-Aldrich.
Results and Discussion
HPLC Screening Strategy: As reflected in the literature and the industry standard, HPLC chiral separation screening remains the most viable and logical approach to identify potential CSP and eluent combinations en route to identifying a needed enantioseparation. Although robust screening can be considered relatively comprehensive in nature, the number of CSPs available is still far greater than what can be feasibly assessed, even in a laboratory dedicated to the process. Screening strategies must be large enough in column capacity to accommodate the more promising CSPs, as well as canvas the various chromatographic modes required by the customer. To complicate matters, a particular CSP can function quite differently between different solvent modes. In some cases, this results in a popular column's presence within more than one screen, while other selected CSPs are screened only in a specific chromatographic mode in which its preferential success has been documented. Finally, fully functional HPLC chiral screens are never static. As new and promising CSPs enter the market, space should be made for evaluation. As a result, a robust state-of-the-art HPLC chiral screen is continuously evolving.
Normal-Phase Screen: As a result of the overall success of polysaccharide CSP and normal-phase eluent combinations for both analytical and preparative-scale enantioseparations, it is not surprising that the normal-phase scheme used in our analytically focused downstream laboratory closely mimics many of the screening systems reported in the literature that support early-phase discovery chemistry operations. If compound solubility and sample matrix are compatible with this separation mode and HPLC columns, the normal-phase polysaccharide screen historically provides the greatest opportunity for early success. Unlike most published normal-phase screens of this type, ours uses a shallow hexane-alcohol gradient, a concept previously reported by de la Puente and colleagues (6) and more recently Zhang (14). Though the standard profile only reaches 30% alcohol in composition, the system frequently discerns low-retentive analytes that might otherwise elute with the solvent front. For the few highly retained compounds not eluted with the shallow gradient, alcohol composition in the eluent is increased in conjunction with a steeper gradient. For some impure samples, a high-to-low alcohol "reverse gradient" is used after the analysis to clean the column. Although this process is not as rapid, perhaps, as other reported normal-phase screens, it seeks to balance speed with quality, minimizing the possibility that an ideal enantioseparation might be missed using a "rapid fire" approach. The list of columns and chromatographic conditions used is provided in Table 1.
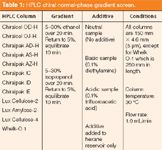
Table 1: HPLC chiral normal-phase gradient screen.
Although the list of CSPs represented above is by no means comprehensive, this normal-phase mode screen has frequently proven successful for identifying the proper columns upon which to direct continued optimization focus. As emphasized earlier, the screen constituent columns are left slightly in flux. New CSPs are also constantly being evaluated.
Polar-Organic Screen: Newly marketed CSPs are also routinely evaluated in the promising polar-organic separation mode. The eluents indicative of this mode (namely short-chain alcohols and acetonitrile) are functional with many of the same columns highlighted in normal-phase screens. Admittedly, the success rate for polar-organic separations is typically less than that observed in an analogous normal-phase screen. Despite this, frequently observed analyte solubility enhancement (for preparative applications) and bandwidth and sensitivity improvements (of analytical interest) realized with polar-organic systems require their investigation. It is of no surprise that polar-organic mode screening is included in many a published screening paradigm (3,5,7,8,10,14). The column and mobile-phase combinations currently used in our laboratory are provided in Table 2.
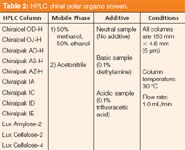
Table 2: HPLC chiral polar organic screen.
Polar-Ionic Screen: The polar-ionic chromatographic mode, an important variation of the popular polar-organic approach, incorporates the addition of combinations of acidic and basic mobile phase additives to polar solvents. Although differentiation between the polar-organic and polar-ionic modes is now relatively straightforward, some earlier literature refers to eluents containing both acidic and basic additives as polar organic, reintroducing confusion. The presence of these competing eluent additives is believed to mostly affect the ionization state of analytes (22). Selected CSPs, namely those using derivatized cyclodextrins or macrocyclic antibiotics, likewise possess ionic sites that are sterically affected when in the presence of these acidic–basic additive combinations. Studies have demonstrated that analytes possessing multiple ionic sites with at least one ionizable point near the stereogenic centre can frequently be resolved using this technique (23). Although the polar solvents of the polar-ionic mode are attractive for preparative chromatography, the occasionally greater amounts of ionic additives (up to 1%), depending on their identity, can cause problems because of potential concentration increases during fraction evaporation. Compared to the more standard polar-organic mode, polar-ionic mode chiral separation screening coverage in the literature is sparse. Huybrechts and colleagues (8) do relate that several chiral analytes separated in their laboratory by both polysaccharide CSPs and macrocyclic antibiotic columns were more efficiently resolved by the latter. The authors additionally offer polar-ionic mode screening as an excellent complementary tool to reversed-phase chiral screening, which is discussed later in this article (8). Multiple polar analytes (notably those containing at least one primary amino and multiple carboxyl ligands) have been successfully resolved in our laboratory using the CSP-mobile phase combinations described in Table 3.
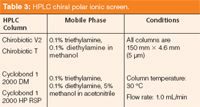
Table 3: HPLC chiral polar ionic screen.
Reversed-Phase Screen: Despite the fact that adequate HPLC chiral separations are many times achieved using the normal-phase, polar-organic, or polar-ionic modes, reversed-phase chromatography can sometimes offer distinct benefits over its nonaqueous counterparts. For analytical laboratories, the practical advantage of an enantioseparation method using solvents miscible with those typically used in traditional reversed-phase achiral HPLC analysis has been mentioned previously. Furthermore, biological samples are aqueous-based, and many pharmaceutical formulations (clinical and toxological) are amenable to hydro-organic sample preparation. Although the sensitivity and expense of the chiral column might still require a degree of sample pretreatment before injection to maximize column lifetime, with reversed-phase separations an otherwise sometimes necessary phase extraction does not complicate the sample preparation process. Concerning the nature of the active ingredient itself, many polar samples are more soluble in aqueous-based solvents. Various authors have touted the advantages of reversed-phase chiral HPLC screens (3,4,8,24,25).
The nature of CSPs represented in our reversed-phase screening paradigm (Table 4) involves three distinct classes; polysaccharide, macrocyclic antibiotic, and cyclodextrin-based. The charge state of the analyte is key to the enantioselective capability of the inherently uncharged polysaccharide CSPs. Enantioselective interaction in the reversed-phase environment with polysaccharide CSPs is usually best when the chiral analyte is in its uncharged state (8). This is sometimes achieved, within column limitations, by eluent pH control. Acidic analytes are screened using a formic acid buffer (adjusted to pH 2), while bases are analyzed with an ammonium bicarbonate buffer (adjusted to pH 9). While other buffer systems are reported in the literature, the volatility of these respective acidic and basic additives allow for liquid chromatography–mass spectrometry (LC–MS) analysis if so desired.

Table 4: HPLC chiral reversed-phase screen.
Because of the limited pH stability of both macrocyclic antibiotic and cyclodextrin CSPs (approximately pH 3 to pH 7), analogous reversed-phase mobile phases of the pH extremes used with the polysaccharide columns is not possible (23). However, this pH limitation is frequently nullified by advantages resulting from alternative separation mechanisms of both the cyclodextrin and macrocyclic antibiotic chiral selector classes. The major retentive and enantioselective interactions realized with cyclodextrins are analyte inclusion complexation within the relatively hydrophobic toroid-shaped cavity and hydrophilic rim attraction (26,27). CSP rim hydroxyl variations allow for a wide variety of choices to affect the desired chiral separation. Two of the more effective and generally reported cyclodextrin-based CSPs are included in our reversed-phase screen.
While macrocyclic antibiotics, like cyclodextrins, possess pockets for analyte inclusion activity, this parameter is much weaker with these selectors. Macrocyclic antibiotics lack the spatial rigidity inherent with the typical cyclodextrin cavity (23). The antibiotics' structures can vary greatly within the relatively narrow pH window in which these CSPs can function. Though the buffer pH within the reversed-phase HPLC screen used is confined to a median pH of 5, the authors realize that optimization of pH with a macrocyclic antibiotic-based reversed-phase chiral separation is a key parameter. Two of the four commercially available macrocyclic antibiotic CSPs are represented in the reversed-phase screen shown in Table 4.
Supercritical Fluid Screening Strategy: To use a robust HPLC multi-modal chiral separation screening operation, without simultaneously addressing the enantioseparation possibilities offered in a comparable SFC-based system, would unnecessarily limit one's investigative options within the modern laboratory. Although chiral chromatography via HPLC still provides the most comprehensive screening because of its applicability across chromatographic modes, SFC is a very powerful option when separation development time and sample analysis speed are paramount. Not surprisingly, drug discovery laboratories have embraced this technology (4,28–30). SFC screens, which typically use a carbon dioxide mobile phase augmented with an alcohol modifier, frequently provide methodology suitable for SFC at the preparative scale. The screen can also guide the researcher towards comparable analytical-scale HPLC conditions in the normal-phase mode or, occasionally, the polar-organic mode.
Although the advantages of rapid screening and lower solvent use inherent with SFC are realized in both preparative and analytical-scale endeavours, instrument complexity and detection limitations remain a challenge to the technique's acceptance in the analytical arena. This mind-set is slowly changing, however, in tandem with improved instrumentation. In the interim, SFC chiral screening data are currently of significant value for rapidly identifying a CSP that will likely analogously separate the enantiomers using more established and rugged HPLC technology. Despite their similarities, the techniques frequently provide differing results, and should be labelled as comparable but complementary (9,10,31–34). In this light, the logic of maintaining a standard normal-phase HPLC chiral screen in the laboratory is justified, especially if the desired end product for downstream development is a rugged HPLC analysis.
Due in large part to the arguments offered above, the SFC screening columns used at any given time in our laboratory closely mirror the constituent columns assigned to the normal-phase HPLC screen. This setup allows for an ongoing assessment of the two unique capabilities over a growing and diverse analyte set. The SFC screen (Table 5), like most in the literature, uses a series of carbon dioxide and alcohol gradients. The gradient is more shallow and lengthy than some, but is focused more on identifying a potential separation (within an acceptable time period) than on speed. As with the normal-phase screen, additives are used for acidic and basic compounds.
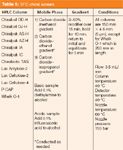
Table 5: SFC chiral screen.
Conclusions
The systematic search for the superior HPLC or SFC chiral separation using various tailored screening systems has become standard practice and is well documented in the literature. Such an approach is effective for quickly identifying promising CSP and eluent combinations that could be optimized to achieve the desired enantioseparation in the appropriate chromatographic mode. Because of the unique and complex interaction mechanisms inherent between an analyte and a given eluent and CSP, total reliance upon predictive databases to identify the optimal enantioseparation system is unlikely. Despite the steady improvement in our understanding of said mechanisms, tailored yet pragmatic chiral separation screening is almost certain to remain the hallmark of the standard pharmaceutical chiral separations laboratory for years to come.
Part 2 of this article covers the establishment of a system suitability plan that focuses on the maintenance of the most important variable in any chiral separation screen: The column. Without reliable testing, regeneration, and maintenance plans in place for the CSPs constituting a screening platform, any data from said system will be of limited value. Once generated, the screening data package is assessed to identify the more promising avenues for enantioseparation optimization and characterization using analytical quality-by-design principles.
Part 2 is available at http://bit.ly/chiralpart2
V. Scott Sharp and Nicole Hicks are with the product design and developability group in drug product development at Eli Lilly and Company in Indianapolis, Indiana, USA. Direct correspondence to: vss@lilly.com
John Stafford is with product and process performance in analytical sciences research and development at Eli Lilly and Company.
References
(1) ChirBase for ACD/Labs, http://www.acdlabs.com/products/adh/chrom/chirbase/ (accessed 17 January 2013).
(2) C. Perrin, V.A. Vu, N. Matthijs, M. Maftouh, D.L. Massart, and Y. Vander Heyden, J. Chromatogr. A. 947, 69–83 (2002).
(3) H. Ates, D. Mangelings, and Y. Vander Heyden, J. Pharm. Biomed. Anal. 48, 288–294 (2008).
(4) D. Mangelings and Y. Vander Heyden, Adv. Chromatogr. 46, 175–210 (2008).
(5) M.E. Andersson, D. Aslan, A. Clarke, J. Roeraade, and G. Hagman, J. Chromatogr. A. 1005, 83–101 (2003).
(6) M.L. de la Puente, C.T. White, A. Rivera-Sagredo, J. Reilly, K. Burton, and G. Harvey, J. Chromatogr. A. 983, 101–114 (2003).
(7) Y. Zhang, W. Watts, L. Nogle, and O. McConnell, J. Chromatogr. A. 1049, 75–84 (2004).
(8) T. Huybrechts, G. Török, T. Vennekens, R. Sneyers, S. Vrielynck, and I. Somers, LCGC Europe 20(6), 320–335 (2007).
(9) M.M. Wong, W.B. Holzheuer, and G.K. Webster, Curr. Pharm. Anal. 4, 101–105 (2008).
(10) P. Franco and T. Zhang, LCGC North Am. 28(9), 818–822 (2010).
(11) T.E. Beesley, LCGC Europe 24(5), 270–276 (2011).
(12) N. Matthijs, M. Maftouh, and Y. Vander Heyden, J. Chromatogr. A. 1111, 48–61 (2006).
(13) B. Chankvetadze, J. Chromatogr. A. 1269, 26–51 (2012).
(14) K.K. Zhang and M.C.Y. Tsang, The Column 8(7), 2–7 (2012).
(15) T. Zhang, D. Nguyen, and P. Franco, J. Chromatogr. A. 1191, 214–222 (2008).
(16) H. Ates, D. Mangelings, and Y. Vander Heyden, in Chiral Separation Methods for Pharmaceutical and Biotechnological Products, S. Ahuja, Ed. (Wiley, Hoboken, New Jersey, USA, 2011), pp. 383–416.
(17) A. Akin, F.J. Antosz, J.L. Ausec, K.F. Greve, R.L. Johnson, L.E. Magnusson, T. Ramstad, S.L. Seacreast, D.S. Seibert, and G.K. Webster, Curr. Pharm. Anal. 3, 53–70 (2007).
(18) Chiral Technologies Chiralpak ID Specification Sheet, http://www.chiraltech.com/spec_sheet/ID_spec_sheet.pdf (accessed Jan. 18, 2013).
(19) Chiral Technologies Chiralpak IE Specification Sheet, http://www.chiraltech.com/spec_sheet/IE_spec_sheet.pdf (accessed Jan. 18, 2013).
(20) Chiral Technologies Chiralpak IF Specification Sheet, http://www.chiraltech.com/spec_sheet/IF_spec_sheet.pdf (accessed Jan. 18, 2013).
(21) R. DePianta, K. Douville, B. Nickerson, and R.E. Borjas, in Chiral Separation Methods for Pharmaceutical and Biotechnological Products, S. Ahuja, Ed. (Wiley, Hoboken, New Jersey, USA< 2011), pp. 209–250.
(22) I. Ali and Y. Aboul-Enein, in Chiral Separation Techniques: A Practical Approach, 3rd ed. G. Subramanian, Ed. (Wiley, Weinheim, Germany, 2007), pp. 21–88.
(23) Advanced Separation Technologies Inc., Chirobiotic Handbook (5th ed), 2004.
(24) C. Perrin, N. Matthijs, D. Mangelings, C. Granier-Loyaux, M. Maftouh, D.L. Massart, and Y. Vander Heyden, J. Chromatogr. A. 966, 119–134 (2002).
(25) T. Zhang, D. Nguyen, and P. Franco, J. Chromatogr. A. 1217, 1048–1055 (2010).
(26) M.H. Hyun, in Chiral Separation Techniques: A Practical Approach, 3rd ed., G. Subramanian, Ed. (Wiley, Weinheim, Germany, 2007), pp. 275–300.
(27) H.Y. Aboul-Enein and I. Ali, Chiral Separations by Liquid Chromatography and Related Technologies (Marcel Dekker, New York, USA, 2003), pp. 281–300.
(28) C. White, J. Chromatogr. A. 1074, 163–173 (2005).
(29) M. Maftouh, C. Granier-Loyaux, E. Chavana, J. Marini, A. Pradines, Y. Vander Heyden, and C. Picard, J. Chromatogr. A. 1088, 67–81 (2005).
(30) T.L. Xiao, M. Wong, and D. Hollowell, "Chiral SFC Column Screening and Overall Chiral Method Development Strategy," presented at Chirality 2012, Fort Worth, Texas, USA, 2012.
(31) K.L. Williams, L.C. Sander, and S.A. Wise, J. Pharm. Biomed. Anal. 15, 1789–1799 (1997).
(32) N. Bargmann-Leyder, A. Tambute', and M. Caude, Chirality 7, 311–325 (1995).
(33) K.L. Williams, L.C. Sander, and S.A. Wise, J. Chromatogr. A. 746, 91–101 (1996).
(34) P. Borman, B Boughtflower, K. Cattanach, K. Crane, K. Freebairn, G. Jonas, I. Mutton, A. Patel, M. Sanders, and D. Thompson, Chirality 15, S1–S12 (2003).

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.










