Monoclonal Antibodies and Biosimilars—A Selection of Analytical Tools for Characterization and Comparability Assessment
Special Issues
This article presents a selection of state-of-the-art analytical tools for mAb characterization and comparability assessment.
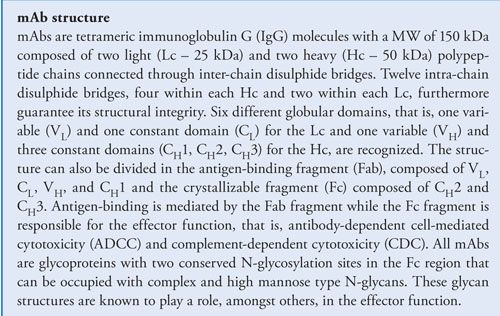
Monoclonal antibodies (mAbs) have emerged as important therapeutics for the treatment of life-threatening diseases including cancer and autoimmune diseases. With the top-selling mAbs evolving out of patent there has been a growing interest in the development of biosimilars. In demonstrating comparability to the originator product, biosimilar developers are confronted with an enormous analytical challenge. This article presents a selection of state-of-the-art analytical tools for mAb characterization and comparability assessment.
It was Paul Ehrlich who, around 1900, reported on “magic bullets” to cure a wide range of diseases, thereby indirectly referring to antibodies (1,2). The development of the hybridoma technology by Köhler and Milstein, which allowed the production of monoclonal antibodies (mAbs), bridged the gap between concept and clinical reality (3). Since the approval of the first therapeutic murine mAb in 1986, advances in antibody engineering have allowed the production of chimeric (mouse–human), humanized, and human monoclonal antibodies, thereby substantially improving safety and efficacy and paving the way for the full exploitation of mAbs for therapeutics purposes (4,5). More than 40 mAbs are now marketed in the United States and Europe for the treatment of a variety of diseases including cancer and autoimmune diseases (6,7).
In 2013, 18 mAbs displayed blockbuster status and six of these products had sales greater than $6 billion (Humira, Remicade, Enbrel, Rituxan, Avastin, and Herceptin). MAbs are currently considered to be the fastest growing class of therapeutics with sales growing from $39 billion in 2008 to almost $75 billion in 2013, a 90% increase. Sales of other recombinant protein biopharmaceuticals have only increased by 26% in the same time period, while small-molecule drugs are stagnating (6,7). The successes of their predecessors have triggered the development of various next-generation mAb formats such as bispecific mAbs, antibody&ndashdrug conjugates (ADC), antibody mixtures, antibody fragments (nanobodies, Fab), Fc fusion proteins, and brain penetrant mAbs next to glyco-engineered formats (4,5,8). With several hundreds of products in preclinical development, the future looks very bright.
The knowledge that the top-selling mAbs are, or will become, open to the market in the coming years has resulted in an explosion of biosimilar activities. Last year witnessed the European approval of the first two monoclonal antibody biosimilars (Remsima and Inflectra), which both contain the same active substance, infliximab (9). Remicade, infliximab’s blockbuster originator, reached global sales of $8.9 billion in 2013. It is clear that the biosimilar market holds great potential, but it is simultaneously confronted with major hurdles. In contrast to generic versions of small molecules, exact copies of recombinant mAbs cannot be produced because of differences in the cell cloning and the manufacturing processes used. Even originator companies experience lot-to-lot variability. As a consequence, regulatory agencies evaluate biosimilars based on their level of similarity to, rather than the exact replication of, the originator. In demonstrating similarity, an enormous weight is placed on analytics, and both the biosimilar and originator need to be characterized and compared in great detail. In contrast to small-molecule drugs, mAbs are large and heterogeneous (as a result of the biosynthetic process and subsequent manufacturing and storage), making their analysis very challenging (10&ndash13). This article reports on selected state-of-the-art chromatographic and mass spectrometric tools for detailed mAb characterization and comparability assessment.
Protein A Chromatography for Clone Selection
Protein A from Staphylococcus aureus has a very strong affinity for the Fc domain of IgG, allowing its capture from complex matrices such as cell culture supernatants. Affinity chromatography making use of Protein A is the gold standard in therapeutic mAb purification and typically represents the first chromatographic step in downstream processing. Protein A chromatography finds applications beyond this large-scale purification. At the analytical scale it is being used early on in the development of mAbs for the high-throughput determination of mAb titer and yield directly from cell culture supernatants and to purify microgram amounts of material for further measurements by techniques such as mass spectrometry (MS) and chromatography (14–16).
Figure 1 shows an overlay of the protein A chromatograms of 12 trastuzumab-producing Chinese hamster ovarian (CHO) clones, generated in the framework of a Herceptin biosimilar development program. Herceptin (scientific INN name trastuzumab) is being used in the treatment of HER2 positive breast cancer. It is open to the European market and evolves out of patent in the United States in 2019 (17). Given its market potential (global sales of $6.5 billion in 2013), dozens of companies are actively developing a Herceptin biosimilar. The unbound CHO material is eluted in the flow-through while the mAb is captured and only released after lowering the pH. From these chromatograms, a distinction can already be made between low and high mAb producing clones. Absolute mAb concentrations can be determined by linking the peak areas to an external calibration curve constructed by diluting Herceptin originators. Obtained mAb titers are visualized in the bar plot in Figure 1. From the findings, clear decisions can be made for further biosimilar development, that is, high trastuzumab producing clones can be selected and taken further in development.
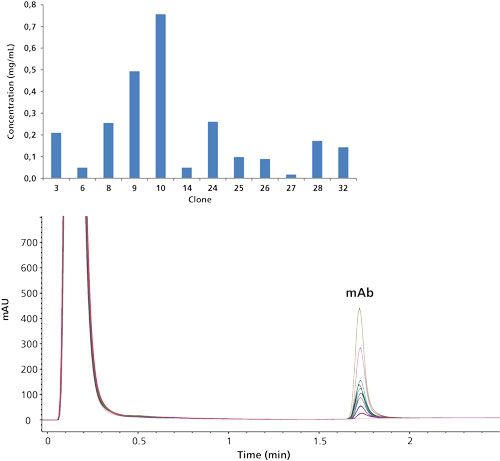
Figure 1: Overlaid UV 280 nm protein A chromatograms of 12 trastuzumab-producing CHO clones with graphical representation of the mAb titer.
Next to the mAb titer, the second important criterion in clone selection is based on the structural aspects. In the case of biosimilar development, the structure should be highly similar to the originator product, within the originator batch-to-batch variations. Figure 2 shows the ion mobility (IM) quadrupole time-of-flight (QTOF) MS measurements of inter-chain reduced Herceptin and protein A–purified trastuzumab from a high titer CHO clone. Samples were introduced into the MS system via a reversed-phase on-line desalting cartridge and light (Lc) and heavy chain (Hc) were resolved in the IM drift cell. Two Lc forms with identical m/z and molecular weight (MW) but with a different drift time, hence conformation, are highlighted. Deconvoluted spectra reveal that clone derived trastuzumab and originator display the same Lc and Hc MW values. In addition, the same N-glycans, which are of the complex type, are observed on the Hc of the originator and clone derived mAb. These are considered the most important attributes of biosimilarity according to US and European regulatory authorities (primary sequence should be identical and glycosylation should be preserved). While glycosylation is similar from a qualitative perspective, quantitative differences are observed. Our experience in clone selection studies has found that it is not always the case that MW values are identical between originators and mAbs derived from high-titer clones or subclones. In these situations, mAbs are typically not taken further in development.

Figure 2: IM-QTOF-MS profile of inter-chain reduced Herceptin (top). Deconvoluted light and heavy chain spectra of a Herceptin originator and a trastuzumab-producing clone (bottom).
Reversed-Phase LC–MS Analysis of Intact, Reduced, Papain, and IdeZ Cleaved mAb
When a mAb is taken further in development, a detailed characterization and comparability assessment has to be performed. Structural characteristics such as amino acid sequence and composition, molecular weight and structural integrity, N- and O-glycosylation, N- and C-terminal processing, S-S bridges, deamidation (asparagine, glutamine), aspartate isomerization, and oxidation (methionine, tryptophan) need to be assessed. In that respect, reversed-phase liquid chromatography (LC) is extremely powerful. Figure 3 shows highly efficient reversed-phase LC–ultraviolet (UV) chromatograms obtained on intact, inter-chain reduced, papain-digested, and nonreduced and reduced IdeZ-cleaved Herceptin. All of these chromatograms are generated using exactly the same chromatographic conditions making use of widepore sub-2-µm C8 particles, elevated column temperatures (80 °C), and trifluoroacetic acid as ion-pairing reagent in a water-acetonitrile mobile phase system. Under these conditions, many of the challenges encountered in performing reversed-phase LC of proteins (peak tailing, peak broadening, and adsorption) are tackled (18-19). Moreover, these conditions are compatible with MS, which allows an in-depth characterization and comparability assessment of mAbs.
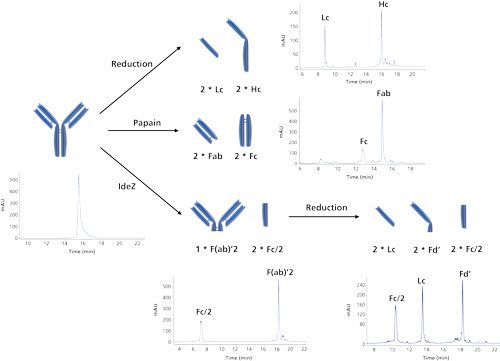
Figure 3: Reversed-phase LC–UV separations of intact, dithiothreitol (DTT)-reduced, papain-digested, nonreduced IdeZ-cleaved and tris(2-carboxyethyl)phosphine (TCEP) reduced IdeZ-cleaved Herceptin. These represent extremely powerful separations for comparability assessment and for detailed characterization. Conditions are compatible with MS allowing identification of the observed peaks.
Figure 4 shows the reversed-phase LC–UV–MS analysis of IdeZ-cleaved and TCEP-reduced Herceptin originator and biosimilar. IdeZ or immunoglobulin-degrading enzyme from Streptococcus equi ssp zooepidemicus is a highly specific protease similar to IdeS that cleaves mAbs at a single site below the hinge region, yielding F(ab′)2 and Fc/2 fragments (20,21). Following reduction, the F(ab′)2 fragment is converted into the Lc and Fd′. From the simultaneously acquired MS data it can be deduced that peaks b, b′, d, d′, g, and g′, corresponding to, respectively, Fc/2, Lc and Fd′, are identical in both the originator and biosimilar. The measured MW values obtained are well below 0.005% of the theoretical MW values, which is expected when using high-resolution and accurate mass instrumentation. Upon examining the spectra of the Fc/2 fragment, the biosimilar appears to be enriched in the N-glycan G0F while a more even distribution between G0F and G1F is observed in the originator. This is also reflected in the chromatographic peak shape. The broader peak b′ indicates a partial separation of the G0F and G1F species, with the former eluting slightly later. Several other differentiating peaks are observed in the separation of the biosimilar, that is, peaks a, c, e, and f. Compared to peak b, peak a displays a 128 Da mass increase, which can be explained by the presence of a C-terminal lysine. To provide some more background on this particular event, the Hc is cloned with a lysine residue at the C-terminus. During protein maturation, this lysine is removed by host cell carboxypeptidases. This process is more dominant in the host cell producing the originator product than in the host cell producing the biosimilar mAb. From the MS data it can be deduced that peak c originates from the Lc plus 1 and 2 hexose units. This potentially originates from a glycation event, which appears to be negligible in the originator mAb. Peak e shows a 1 Da mass increase compared to peak d, indicating a deamidation in the Lc. This event is apparent in both the originator and biosimilar with an increased occurrence in the biosimilar. In analogy with peak c, peak f displays 162 Da spacings on Fd′, which is indicative of glycation.
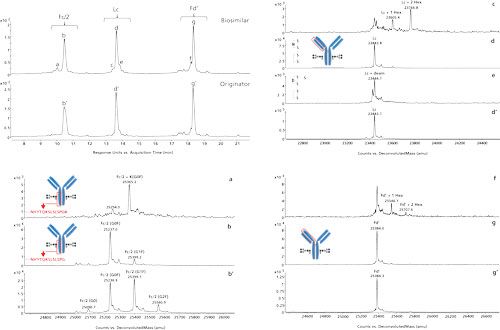
Figure 4: Reversed-phase LC–UV–MS analysis of IdeZ-cleaved and TCEP-reduced Herceptin originator and biosimilar and deconvoluted MS spectra associated with the annotated peaks.
Reversed-Phase LC–MS Analysis for Peptide Mapping
As previously demonstrated, protein measurement is extremely powerful but does not provide the complete picture. While it is indicative for identity and highlights dominant modifications, it does not provide the actual amino acid sequence nor does it localize the modifications. For example, the measurement presented in Figure 4 reveals a deamidation on the Lc (peak e) but it cannot be traced back to a specific asparagine or glutamine residue. The Lc of the measured mAb contains six asparagine and 15 glutamine residues, which are all prone to this chemical modification. These characteristics can further be assessed at the peptide level following proteolytic digestion. When digesting Herceptin with the enzyme trypsin, which cleaves the protein next to arginine and lysine residues, 62 identity peptides are formed. Taking into account post-translational modifications and incomplete and aspecific cleavages taking place, more than 100 peptides with varying physicochemical properties in a wide dynamic concentration can be expected. This is a particularly complex sample and demands the best in terms of separation technique. Again, reversed-phase LC is the method of choice to resolve these complex mixtures. Figure 5 shows the UV peptide maps of both the originator and biosimilar. By taking advantage of the simultaneously acquired MS data, more than 99% of the peptide sequence can be covered in both the originator and biosimilar thereby confirming identity. While peptide maps are highly comparable, differences in post-translational modifications can be detected (Figure 5). Obtaining a good knowledge of all of these modifications is important since they could be critical to the potency and safety of a mAb. A deviating glycosylation profile between originator and biosimilar is already revealed at the protein level (Figure 4). At the peptide level, the different N-glycosylated variants are nicely resolved chromatographically and are shown to be located on peptide EEQYNSTYR. Again, the undergalactosylation of the biosimilar is apparent. The peptide map also reveals the presence of a lysine at the C-terminal peptide of the heavy chain (SLSLSPGK) and slightly increased deamidation in a light chain peptide (ASQDVNTAVAWYQQK). This particular peptide contains four potential deamidation sites (3 Gln and 1 Asn). Based on MS measurement one cannot discriminate between the four sites. Upon performing MS/MS and carefully interpreting the fragment ions observed, the deamidation can be traced back to the N (11). This deamidation in fact corresponds to the deamidation event observed in the reduced IdeS digest (Figure 4). At that time this event could be linked to the Lc but could not be traced back to a specific residue.
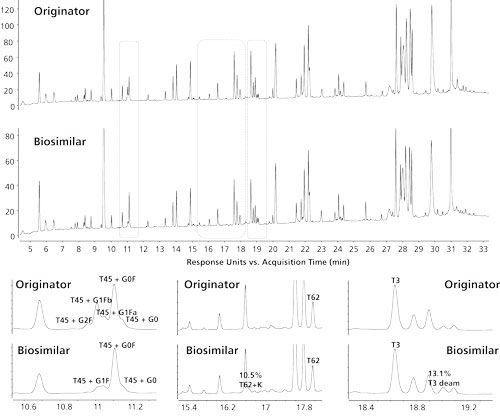
Figure 5: Reversed-phase LC–UV peptide map of Herceptin originator and biosimilar with detail in some specific regions showing post-translational modifications. T45: EEQYNSTYR, T62: SLSLSPG, T3: ASQDVNTAVAWYQQK. Peak identities were assigned by the simultaneously acquired MS and MS/MS data.
As discussed, mAb digests can be quite complex and their analysis demands the best in terms of separating power. If one-dimensional (1D) separations are not able to provide the separation power needed, one can opt for two-dimensional (2D) LC. Compared to 1D-LC, 2D-LC and especially comprehensive LC (LC×LC) will drastically increase resolution. We have recently described the analysis of Herceptin originator and biosimilar digests on the combination reversed-phase LC×reversed-phase LC (22). It is important to point out that orthogonality in reversed-phase LC×reversed-phase LC peptide mapping is only obtained when operating the two reversed-phase LC columns at different pH values. This is a direct result of the zwitterionic nature of peptides, which gives rise to major selectivity differences at pH extremes. These reversed-phase LC×reversed-phase LC peptide maps provide a wealth of information and allow both identity and purity to be assessed. This makes it an attractive technology for the comparison of different production batches and to compare innovator biopharmaceuticals with biosimilars.
Native Chromatographic Tools: Size-Exclusion Chromatography, Cation-Exchange Chromatography, and Hydrophobic Interaction Chromatography
In contrast to reversed-phase LC, size-exclusion chromatography (SEC), ion-exchange chromatography, and hydrophobic interaction chromatography (HIC) are nondenaturing techniques that provide complementary information to the afore-mentioned chromatographic mode (Figure 6). These techniques are used early on in mAb characterization and comparability assessment and are subsequently applied in routine testing. A major advantage of these chromatographic modes compared to reversed-phase LC is that they preserve the structure, and so minor variants can be collected and subjected to complementary techniques such as potency determination. SEC, ion-exchange chromatography, and HIC are not directly compatible with MS because of the presence of nonvolatile salts in the mobile phases. The identification of peaks requires their collection and subsequent desalting or dilution before MS measurement. Desalting of the collected fractions can be performed in an automated manner using a setup such as a small reversed-phase cartridge hyphenated to an MS system.
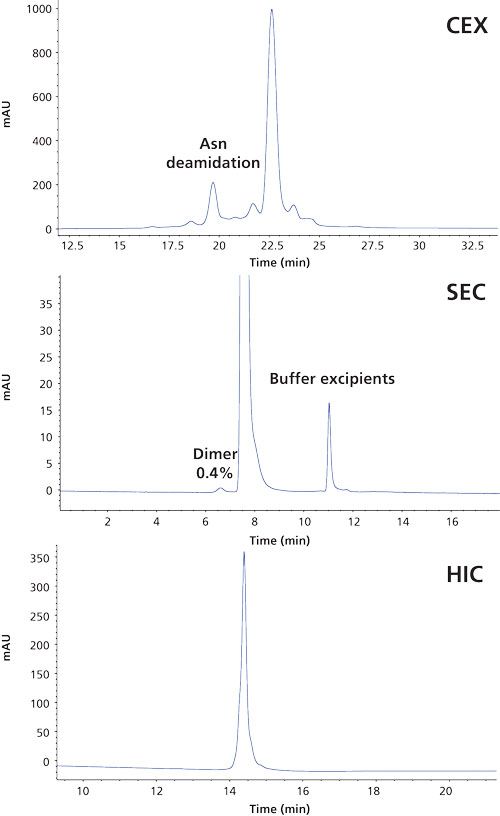
Figure 6: CEX, SEC, and HIC separations of Herceptin. These techniques are used in characterization and release testing.
Ion-exchange chromatography is an excellent tool to highlight charged variants that might arise from modifications such as deamidation, lysine truncation, or N-terminal cyclization (23,24). Since most therapeutic mAbs have a higher proportion of basic residues, cation-exchange chromatography is the most commonly used technique. The cation-exchange separation of Herceptin (Figure 6) highlights the asparagine deamidation discussed earlier. A deamidation renders a protein more acidic, which explains this earlier elution.
SEC is the chromatographic mode with the lowest efficiency or resolution of the afore-mentioned techniques, but it is extremely powerful when determining aggregation and fragmentation. It is recognized that aggregates may stimulate immune responses and it is therefore very important to measure this critical quality attribute. Aggregation can typically not be assessed using the other chromatographic modes discussed. Figure 6 presents the SEC analysis of Herceptin and illustrates that dimers can be measured accurately at levels as low as 0.4%.
In recent years, HIC has been revisited mainly from the perspective of ADC’s governing a separation based on the number of conjugated drugs allowing the drug-to-antibody ratio (DAR) to be determined. In the separation of naked mAbs it is useful to highlight heterogeneities originating from oxidation, aspartate isomerization, deamidation, succinimide formation, C-terminal lysine, and clipping. The HIC analysis of Herceptin gives rise to a single chromatographic peak (Figure 6).
Hydrophilic Interaction Liquid Chromatography for Glycan Profiling
As demonstrated, glycosylation can be revealed at both the protein and peptide level. A detailed insight into the sugars, however, can only be obtained following their removal from the protein–peptide backbone. This is preferably done enzymatically using the deglycosidase PNGase F. The liberated sugars are subsequently labeled via reductive amination to improve their chromatographic separation and detectability (fluorescence or mass spectrometric detection). The fluorescence trace is typically used for quantitative purposes while the MS trace is used for qualitative purposes. Figure 7a displays the analysis of 2-aminobenzamide (2-AB) labelled Herceptin originator and biosimilar N-glycans using hydrophilic interaction chromatography (HILIC) with fluorescence detection (FLD). In this particular case, a column packed with superficially porous HILIC particles compatible with 600 bar high performance liquid chromatography (HPLC) instrumentation was used. This measurement provides information on the glycans and allows structural isomers, that is, G1Fa and G1Fb which differ in the positioning of the galactose residue either on the α1-3 or α1-6 branch of the complex type glycan, to be resolved.
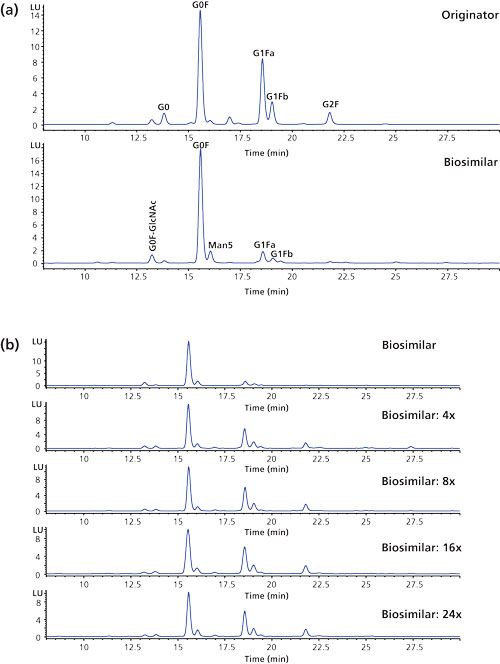
Figure 7: (a) Overlaid HILIC-FLD chromatograms of the 2-AB labeled N-glycans enzymatically released from Herceptin originator and Protein A purified biosimilar. (b) N-glycan profiles of the biosimilar obtained by growing the CHO clone at different galactose, uridine, and manganese chloride concentrations. Separations were performed on superficially porous HILIC particles.
The same type of complex N-glycans are observed on both the originator and biosimilar but quantitative differences are revealed with an overexpression of G0F species on the biosimilar, which is in accordance with the measurements performed at protein and peptide level. Since glycosylation is a critical quality attribute, this undergalactosylation does not make the product similar enough to be considered by regulatory authorities as a Herceptin biosimilar.
The biosimilar-producing CHO cell culture medium was subsequently tuned by feeding uridine (U), galactose (G), and manganese chloride (M) at different concentrations (25). These are the substrates and activator of the galactosyltransferase responsible for donating galactose residues to G0F and G1F acceptors. Figure 7b shows the N-glycan profiles obtained by growing the biosimilar producing CHO clone at different U, G, and M concentrations. It is observed that the ratio G1F–G0F increases with increasing concentration of U, G, and M. From these results it can be concluded that conditions can be found that allow the glycosylation of the biosimilar to fit within the originator specifications.
Conclusion
In the development of biosimilars, a comprehensive comparability exercise involving the originator product is required to demonstrate similarity in terms of physicochemical characteristics, efficacy, and safety. In that respect, an enormous weight is placed on analytics and the analytical package for a biosimilar mAb submission is considerably larger than that of a stand-alone mAb. Structural differences define the amount of preclinical and clinical studies required. A wide range of analytical tools providing complimentary information is available to guide biosimilar development.
Acknowledgments
The authors acknowledge Maureen Joseph (Agilent Technologies, Wilmington, Delaware), David Wong (Agilent Technologies, Santa Clara, California) and Lindsay Mesure (Promega, Leiden, The Netherlands).
References
- K. Strebhardt and A. Ullrich, Nat. Rev. Cancer8, 473–480 (2008).
- L.M. Weiner, R. Surana, and S. Wang, Nat. Rev. Immunol.10, 317–327 (2010).
- G. Köhler and C. Milstein, Nature256, 495–497 (1975).
- N.A.P.S. Buss, S.J. Henderson, M. McFarlane, J.M. Shenton, and L. de Haan, Curr. Opin. Pharmacol.8, 620–626 (2008).
- J.G. Elvin, R.G. Couston, and C.F. van der Walle, Int. J. Pharm. 440, 83–98 (2013).
- D.M. Ecker, S.D. Jones, and H.L. Levine, mAbs7, 9–14 (2015).
- G. Walsh, Nat. Biotechnol.32, 992–1000 (2014).
- G. Walsh, Nat. Biotechnol. 28, 917–924 (2010).
- A. Beck and J.M. Reichert, mAbs 5, 621–623 (2013).
- K. Sandra, I. Vandenheede, and P. Sandra, J. Chromatogr. A 1335, 81–103 (2014).
- K. Sandra, I. Vandenheede, and P. Sandra, LCGC Europe May Supplement, 10–16 (2013).
- A. Beck, S. Sanglier-Cianférani, and A. Van Dorsselaer, Anal. Chem.84, 4637–4646 (2012).
- A. Beck, E. Wagner-Rousset, D. Ayoub, A. Van Dorsselaer, and S. Sanglier-Cianférani, Anal. Chem.85, 715–736 (2013).
- E. Dumont, I. Vandenheede, P. Sandra, K. Sandra, J. Martosella, P. Duong, M. Joseph, Agilent Technologies Application Note 5991-5124EN (2014).
- E. Dumont, I. Vandenheede, P. Sandra, K. Sandra, J. Martosella, P. Duong, M. Joseph, Agilent Technologies Application Note 5991-5125EN (2014).
- E. Dumont, I. Vandenheede, P. Sandra, K. Sandra, J. Martosella, P. Duong, M. Joseph, Agilent Technologies Application Note 5991-5135EN (2014).
- A. Staub, D. Guillarme, J. Schappler, J.L. Veuthey, and S. Rudaz, J. Pharm. Biomed. Anal.55, 810–822 (2011).
- S. Fekete, J.L. Veuthey, and D. Guillarme, J. Pharm. Biomed. Anal. 69, 9–27 (2012).
- G. Chevreux, N. Tilly, and N. Bihoreau, Anal. Biochem.415, 212–214 (2011).
- C. Hosfield, P. Compton, L. Fornelli, P. Thomas, N.L. Kelleher, M. Rosenblatt, and M. Urh, Promega Poster Part#PS260 (2015).
- G. Vanhoenacker, I. Vandenheede, F. David, P. Sandra, and K. Sandra, Anal. Bioanal. Chem.407, 355–366 (2015).
- I. Vandenheede, E. Dumont, P. Sandra, K. Sandra, M. Joseph, Agilent Technologies Application Note 5991-5273EN (2014).
- I. Vandenheede, E. Dumont, P. Sandra, K. Sandra, M. Joseph, Agilent Technologies Application Note 5991-5274EN (2014).
- M.J. Gramer, J.J Eckblad, R. Donahue, J. Brown, C. Schultz, K. Vickerman, P. Priem, E.T. van den Bremern J. Gerritsen, and P.H. van Berkel, Biotechnol. Bioeng. 108, 1591–1602 (2011).
Koen Sandra is Director at the Research Institute for Chromatography (RIC) in Kortrijk, Belgium.
Isabel Vandenheede is a Protein Analyst at the Research Institute for Chromatography (RIC).
Emmie Dumont is an LC-MS Specialist at the Research Institute for Chromatography (RIC).
Pat Sandra is Chairman at the Research Institute for Chromatography (RIC) and Emeritus Professor at Ghent University in Ghent, Belgium.

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










